Chemistry:Colitose
| |
| |
| Names | |
|---|---|
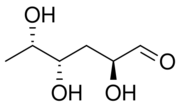
| IUPAC name
(2S,4S,5S)-2,4,5-Trihydroxyhexanal
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H12O4 | |
| Molar mass | 148.158 g·mol−1 |
| Density | 1.25 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Colitose is a mannose-derived 3,6-dideoxysugar produced by certain bacteria. It is a constituent of the lipopolysaccharide.[1] It is the enantiomer of abequose.
Biological role
Colitose is found in the O-antigen of certain Gram-negative bacteria such as Escherichia coli, Yersinia pseudotuberculosis, Salmonella enterica, Vibrio cholerae, and in marine bacteria such as Pseudoalteromonas sp.[1][2] The sugar was first isolated in 1958,[3] and subsequently was enzymatically synthesized in 1962.[4]
Biosynthesis
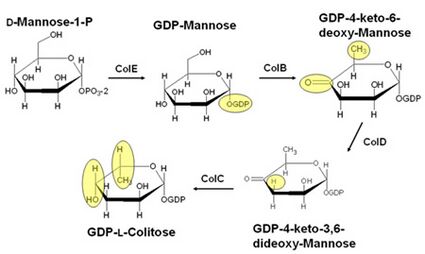
The biosynthesis of colitose begins with ColE, a mannose-1-phosphate guanylyltransferase that catalyzes the addition of a GMP moiety to mannose, yielding GDP-mannose. In the next step, ColB, an NADP-dependent short-chain dehydrogenase-reductase enzyme, catalyzes the oxidation at C-4 and the removal of the hydroxyl group at C-6. The resulting product, GDP-4-keto-6-deoxymannose, then reacts with the PLP-dependent enzyme GDP-4-keto-6-deoxymannose-3-dehydratase (ColD), which removes the hydroxyl at C-3 in a manner similar to that of serine dehydratase. In the final step, the product of ColD, GDP-4-keto-3,6-dideoxymannose, reacts with ColC, which reduces the ketone functionality at C-4 back to an alcohol and inverts the configuration about C-5.[5]
The resulting product, GDP-L-colitose, is then incorporated into the O-antigen by glycosyltransferases and O-antigen processing proteins. Further reactions join the O-antigen to the core polysaccharide to form the full lipopolysaccharide.
GDP-4-keto-6-deoxymannose-3-dehydratase (ColD)
ColD is a PLP-dependent enzyme responsible for the removal of the C-3' hydroxyl group during the biosynthesis of GDP-colitose.[5] It is a product of the Wbdk or ColD genes in Escherichia coli O55 or Salmonella enterica, respectively, and is commonly referred to as ColD.[1]
Usage in biotechnology
Although the sugar is relatively rare, recent work with glycosyltransferases suggests that obscure sugars such as colitose can be incorporated into existing natural-product scaffolds, thereby constructing novel and potentially therapeutic compounds.[6]
References
- ↑ 1.0 1.1 1.2 "Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly". Carbohydrate Research 338 (23): 2503–19. November 2003. doi:10.1016/j.carres.2003.07.009. PMID 14670712.
- ↑ "Structure of a colitose-containing O-specific polysaccharide of the marine bacterium Pseudoalteromonas tetraodonis IAM 14160(T)". Carbohydrate Research 333 (1): 41–6. June 2001. doi:10.1016/S0008-6215(01)00121-5. PMID 11423109.
- ↑ "[Colitose, 3-desoxy-L-fucose, a new sugar building block with immunospecific functions in the endotoxins, lipopolysaccharides, of some gram-negative bacteria.]" (in de). Biochemische Zeitschrift 330 (3): 193–7. 1958. PMID 13546193.
- ↑ "The enzymatic synthesis of guanosine diphosphate colitose by a mutant strain of Escherichia coli". Proceedings of the National Academy of Sciences of the United States of America 48 (7): 1209–16. July 1962. doi:10.1073/pnas.48.7.1209. PMID 13905784.
- ↑ 5.0 5.1 "Biosynthesis of colitose: expression, purification, and mechanistic characterization of GDP-4-keto-6-deoxy-D-mannose-3-dehydrase (ColD) and GDP-L-colitose synthase (ColC)". Biochemistry 43 (51): 16450–60. December 2004. doi:10.1021/bi0483763. PMID 15610039.
- ↑ "Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions". Science 313 (5791): 1291–4. September 2006. doi:10.1126/science.1130028. PMID 16946071.
 |