Biology:Flagellar motor switch protein
| FliG C-terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
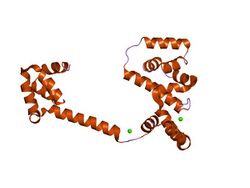 crystal structure of the middle and c-terminal domains of the flagellar rotor protein flig | |||||||||
| Identifiers | |||||||||
| Symbol | FliG_C | ||||||||
| Pfam | PF01706 | ||||||||
| Pfam clan | CL0436 | ||||||||
| InterPro | IPR000090 | ||||||||
| SCOP2 | 1qc7 / SCOPe / SUPFAM | ||||||||
| |||||||||
| Flagellar motor switch protein FliM | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | FliM | ||||||||
| Pfam | PF02154 | ||||||||
| Pfam clan | CL0355 | ||||||||
| InterPro | IPR001689 | ||||||||
| |||||||||
In molecular biology, the flagellar motor switch protein (Flig) is one of three proteins in certain bacteria coded for by the gene fliG.[1] The other two proteins are FliN coded for by fliN,[2] and FliM coded for by fliM.[3] The protein complex regulates the direction of flagellar rotation and hence controls swimming behaviour.[4] The switch is a complex apparatus that responds to signals transduced by the chemotaxis sensory signalling system during chemotactic behaviour.[4] CheY, the chemotaxis response regulator, is believed to act directly on the switch to induce a switch in the flagellar motor direction of rotation.
Fli proteins
The switch complex comprises at least three proteins - FliG, FliM and FliN.[2] It has been shown that FliG interacts with FliM, FliM interacts with itself, and FliM interacts with FliN.[5] Several amino acids within the middle third of FliG appear to be strongly involved in the FliG-FliM interaction, with residues near the N- or C-termini being less important.[5] Such clustering suggests that FliG-FliM interaction plays a central role in switching.
Analysis of the FliG, FliM and FliN sequences shows that none are especially hydrophobic or appear to be integral membrane proteins.[6] This result is consistent with other evidence suggesting that the proteins may be peripheral to the membrane, possibly mounted on the basal body M ring.[6][7] FliG is present in about 25 copies per flagellum. The structure of the C-terminal domain of FliG is known, this domain functions specifically in motor rotation.[8]
References
- ↑ "flig in UniProtKB". https://www.uniprot.org/uniprot/?query=flig&sort=score.
- ↑ 2.0 2.1 "fliN - Flagellar motor switch protein FliN - Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720) - fliN gene & protein". https://www.uniprot.org/uniprot/P26419.
- ↑ "flim in UniProtKB". https://www.uniprot.org/uniprot/?query=FliM&sort=score.
- ↑ 4.0 4.1 "Gene sequence, overproduction, purification and determination of the wild-type level of the Escherichia coli flagellar switch protein FliG". Gene 133 (1): 103–8. October 1993. doi:10.1016/0378-1119(93)90232-R. PMID 8224881.
- ↑ 5.0 5.1 "A mutational analysis of the interaction between FliG and FliM, two components of the flagellar motor of Escherichia coli". J. Bacteriol. 178 (5): 1289–94. March 1996. doi:10.1128/jb.178.5.1289-1294.1996. PMID 8631704.
- ↑ 6.0 6.1 "Flagellar switch of Salmonella typhimurium: gene sequences and deduced protein sequences". J. Bacteriol. 171 (6): 3247–57. June 1989. doi:10.1128/jb.171.6.3247-3257.1989. PMID 2656645.
- ↑ "Localization of the Salmonella typhimurium flagellar switch protein FliG to the cytoplasmic M-ring face of the basal body". Proc. Natl. Acad. Sci. U.S.A. 89 (14): 6304–8. July 1992. doi:10.1073/pnas.89.14.6304. PMID 1631122. Bibcode: 1992PNAS...89.6304F.
- ↑ "Structure of the C-terminal domain of FliG, a component of the rotor in the bacterial flagellar motor". Nature 400 (6743): 472–5. July 1999. doi:10.1038/22794. PMID 10440379. Bibcode: 1999Natur.400..472L.
 |

