Chemistry:Alpha-Eleostearic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
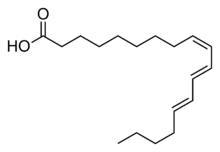
(9Z,11E,13E)-Octadeca-9,11,13-trienoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| 1726551 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H30O2 | |
| Molar mass | 278.43 g/mol |
| Melting point | 48 °C (118 °F; 321 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
α-Eleostearic acid or (9Z,11E,13E)-octadeca-9,11,13-trienoic acid, is an organic compound, a conjugated fatty acid and one of the isomers of octadecatrienoic acid. It is often called simply eleostearic acid although there is also a β-eleostearic acid (the all-trans or (9E,11E,13E) isomer). Its high degree of unsaturation gives tung oil its properties as a drying oil.
Biochemical properties
In their pioneering work on essential fatty acids, Burr, Burr and Miller compared the nutritional properties of α-eleostearic acid (ELA) to that of its isomer alpha-linolenic acid (ALA). ALA relieved essential fatty acid deficiency; ELA did not.[1]
In rats, α-eleostearic acid is converted to a conjugated linoleic acid.[2] The compound has been found to induce programmed cell death of fat cells,[3] and of HL60 leukemia cells in vitro at a concentration of 20 μM.[4] Diets containing 0.01% bitter gourd seed oil (0.006% as α-eleostearic acid) were found to prevent azoxymethane-induced colon carcinogenesis in rats.[5]
Sources
α-Eleostearic acid is found in the oils extracted from seeds. Tung oil has 82% α-eleostearic acid. Bitter gourd seed oil has 60% α-eleostearic acid.
Etymology
Eleo- is a prefix derived from the Greek word for olive, ἔλαιον.[6]
See also
References
- ↑ 1.0 1.1 Burr, G.O.; Burr, M.M.; Miller, E. (1932). "On the nature and role of the fatty acids essential in nutrition". J. Biol. Chem. 97 (1): 1–9. http://www.jbc.org/cgi/reprint/97/1/1.pdf. Retrieved 2007-01-17.
- ↑ "Conjugated linolenic acid is slowly absorbed in rat intestine, but quickly converted to conjugated linoleic acid". J Nutr 136 (8): 2153–9. 1 August 2006. doi:10.1093/jn/136.8.2153. PMID 16857834.
- ↑ "Regulation of apoptosis through arachidonate cascade in mammalian cells". Appl Biochem Biotechnol 102–103 (1–6): 239–50. 2002. doi:10.1385/ABAB:102-103:1-6:239. PMID 12396127.
- ↑ "α-Eleostearic Acid and Its Dihydroxy Derivative Are Major Apoptosis-Inducing Components of Bitter Gourd". Journal of Agricultural and Food Chemistry 56 (22): 10515–10520. 2008. doi:10.1021/jf8020877. PMID 18959405.
- ↑ "Dietary seed oil rich in conjugated linolenic acid from bitter melon inhibits azoxymethane-induced rat colon carcinogenesis through elevation of colonic PPAR γ expression and alteration of lipid composition.". International Journal of Cancer 110 (6): 896–901. 2004. doi:10.1002/ijc.20179. PMID 15170673.
- ↑ Senning, Alexander (2006-10-30). Elsevier's Dictionary of Chemoetymology: The Whys and Whences of Chemical Nomenclature and Terminology. ISBN 9780080488813. https://books.google.com/books?id=Fl4sdCYrq3cC&q=eleo.


