Chemistry:Dichlorobis(ethylenediamine)nickel(II)
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C8H32Cl4N8Ni2 | |
| Molar mass | 499.59 g·mol−1 |
| Density | 1.66 g/cm3 |
| Melting point | > (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Dichlorobis(ethylenediamine)nickel(II) is the inorganic compound with the formula NiCl2(en)2, where en = ethylenediamine. The formula is deceptive: the compound is the chloride salt of the coordination complex [Ni2Cl2(en)4]2+. This blue solid is soluble in water and some polar organic solvents. It is prepared by ligand redistribution from [Ni(en)3]Cl2 • 2H2O and hydrated nickel chloride:[1]
- 2 [Ni(en)3]Cl2 + NiCl2 → 3 NiCl2(en)2
The rapid ligand redistribution is characteristic of the kinetic lability of octahedral nickel(II) complexes. In contrast with the lability of [Ni(en)3]Cl2+ is the inertness of the isostructural tris(ethylenediamine)cobalt(III) cation.
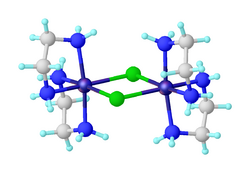
Structure of the dication [Ni2Cl2(en)4]2+ that comprises the salt "NiCl2(en)2".[2]
References
- ↑ State, Harold M. (1960). "Bis(Ethylenediamine)Nickel(II) Chloride". Inorganic Syntheses 6: 198–199. doi:10.1002/9780470132371.ch63.
- ↑ Joung, K. O.; O'Connor, C. J.; Sinn, E.; Carlin, R. L. (1979). "Structural and magnetic properties of dimeric di-.mu.-chloro-tetrakis(ethylenediamine)dinickel(II) chloride". Inorganic Chemistry 18 (3): 804–808. doi:10.1021/ic50193a054.
 |

