Chemistry:Oxalyl dicyanide
From HandWiki
Revision as of 13:03, 30 June 2021 by imported>CodeMe (linkage)
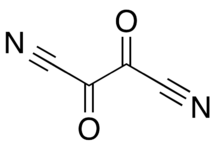
| |
| Names | |
|---|---|
| Preferred IUPAC name
Oxalyl dicyanide[1] | |
| Systematic IUPAC name
Ethanedioyl dicyanide | |
| Other names
Oxalyl cyanide
Ethanedioyl cyanide Dioxosuccinonitrile 2,3-Doxosuccinonitrile Dioxobutanedinitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4N2O2 | |
| Molar mass | 108.05 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oxalyl dicyanide is a chemical compound with the formula C4N2O2.
Formation
Oxalyl dicyanide can be formed by the hydrolysis of diiminosuccinonitrile.[2]
Reactions
Oxalyl dicyanide can condense with diaminomaleonitrile to make pyrazinetetracarbonitrile and also 5,6-dihydroxypyrazine-2,3-dicarbonitrile, both derivatives of pyrazine.[2]
See also
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 902. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 (in en) Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 16: Six-Membered Hetarenes with Two Identical Heteroatoms. Georg Thieme Verlag. 2014. p. 2035. ISBN 978-3-13-178071-3. https://books.google.com/books?id=ruWIAwAAQBAJ&pg=RA1-PA2035.
 |

