Chemistry:Tetraphenyl butadiene
From HandWiki
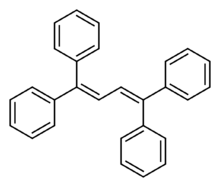
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,1′,1′′,1′′′-(Buta-1,3-diene-1,1,4,4-tetrayl)tetrabenzene | |
| Other names
TPB
| |
| Identifiers | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C28H22 | |
| Molar mass | 358.484 g·mol−1 |
| Appearance | White to yellow white needles |
| Density | 1.079 g/cm3 |
| Melting point | 203.5 °C (398.3 °F; 476.6 K) |
| Solubility | soluble in ethanol, benzene, chloroform, acetic acid[1] |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 289 °C (552 °F; 562 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetraphenyl butadiene (1,1,4,4-tetraphenyl-1,3-butadiene or TPB) is an organic chemical compound used as an electroluminescent dye. It glows blue with an emission spectrum peak wavelength at 430 nm,[3] which makes it useful as a wavelength shifter.[4][5]
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 3–526. ISBN 978-0-8493-0594-8.
- ↑ "1,1,4,4-Tetraphenyl-1,3-butadiene" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/74060#section=Safety-and-Hazards.
- ↑ Burton, W. M; Powell, B. A (1973). "Fluorescence of Tetraphenyl-Butadiene in the Vacuum Ultraviolet". Applied Optics 12 (1): 87–9. doi:10.1364/AO.12.000087. PMID 20125234. Bibcode: 1973ApOpt..12...87B..
- ↑ Wise, Donald Lee; Gary E. Wnek; Debra J. Trantolo; Thomas M. Cooper; Joseph D. Gresser (1998). Photonic Polymer Systems. CRC Press. p. 250. ISBN 978-0-8247-0152-9. https://books.google.com/books?id=CseEUIK6dVkC&q=%221,1,4,4-Tetraphenyl-1,3-butadiene%22&pg=PA250. Retrieved 2009-06-02.
- ↑ Wernick, Miles N.; John N. Aarsvold (2004). Emission Tomography. Academic Press. p. 374. ISBN 978-0-12-744482-6. https://books.google.com/books?id=R5slur_hdfEC&q=%22Tetraphenyl+butadiene%22&pg=PA11. Retrieved 2009-06-02.
 |


