Chemistry:Trypan blue
| |
| Names | |
|---|---|
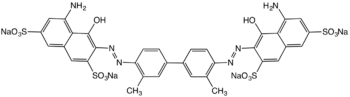
| IUPAC name
(3Z,3'Z)-3,3'-[(3,3'-dimethylbiphenyl-4,4'-diyl)di(1Z)hydrazin-2-yl-1-ylidene]bis(5-amino-4-oxo-3,4-dihydronaphthalene-2,7-disulfonic acid)
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C34H24N6Na4O14S4 | |
| Molar mass | 872.88 |
| Appearance | deep blue in aqueous solution[1] |
| Melting point | > 300 °C (572 °F; 573 K) |
| <0.1 mg/mL in water [2] | |
| Solubility | 20 mg/mL in methyl Cellosolve, and 0.6 mg/mL in ethanol |
| Pharmacology | |
| 1=ATC code }} | S01KX02 (WHO) |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
6200 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
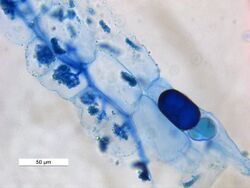
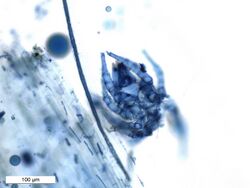
Trypan blue is an azo dye. It is a direct dye for cotton textiles.[3] In biosciences, it is used as a vital stain to selectively colour dead tissues or cells blue.
Live cells or tissues with intact cell membranes are not coloured. Since cells are very selective in the compounds that pass through the membrane, in a viable cell trypan blue is not absorbed; however, it traverses the membrane in a dead cell. Hence, dead cells appear as a distinctive blue colour under a microscope. Since live cells are excluded from staining, this staining method is also described as a dye exclusion method.
Background and chemistry
Trypan blue is derived from toluidine, that is, any of several isomeric bases, C14H16N2, derived from toluene. Trypan blue is so-called because it can kill trypanosomes, the parasites that cause sleeping sickness. An analog of trypan blue, suramin, is used pharmacologically against trypanosomiasis. Trypan blue is also known as diamine blue and Niagara blue.
The extinction coefficient for trypan blue is 6⋅104 M−1 cm−1 at 607 nm in methanol.[4]
Trypan red and trypan blue were first synthesized by the German scientist Paul Ehrlich in 1904.
Uses of trypan blue
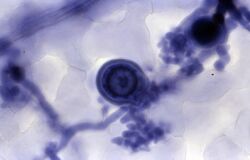
Trypan blue is commonly used in microscopy (for cell counting) and in laboratory mice for assessment of tissue viability.[5] The method cannot distinguish between necrotic and apoptotic cells.
It may be used to observe fungal hyphae[6] and stramenopiles.
Trypan blue is also used in ophthalmic cataract surgery to stain the anterior capsule in the presence of a mature cataract, to aid in visualization, before creating the continuous curvilinear capsulorhexis. In keratoplasty, trypan blue can be used to stain the posterior stromal fibers during deep lamellar endothelial keratoplasty (DLEK) and to stain the endothelium in Descemet's stripping endothelial keratoplasty (DSEK). Trypan blue is used in vitreoretinal surgeries also.[7]
In early 20th century, the existence of a barrier protective toward the brain (blood brain barrier) was inferred, based on the observation that injection of trypan blue in animals led to whole‐body staining except for the brain and spinal cord.
Synonyms
- Azidine blue 3B
- Benzamine blue 3B
- Benzo Blue bB
- Chlorazol blue 3B
- Diamine blue 3B
- Dianil blue H3G
- Direct blue 14
- Niagara blue 3B
Further reading
- Chapter "Detection of Caspase Activation Combined with Other Probes of Apoptosis", Eurekah Bioscience Collection, NCBI bookshelf
- Protocol for use of the dye (PDF) from Northwestern University
- Wainwright, M. (December 2010). "Dyes, trypanosomiasis and DNA: a historical and critical review". Biotechnic & Histochemistry 85 (6): 341–54. doi:10.3109/10520290903297528. PMID 21080764.
References
- ↑ PubChem. "CID 9562061 | C34H24N6Na4O14S4 - PubChem". Pubchem.ncbi.nlm.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/9562061#section=Color. Retrieved 2022-04-19.
- ↑ PubChem. "CID 9562061 | C34H24N6Na4O14S4 - PubChem". Pubchem.ncbi.nlm.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/9562061#section=Solubility. Retrieved 2022-04-19.
- ↑ Klaus Hunger; Peter Mischke; Wolfgang Rieper; Roderich Raue; Klaus Kunde; Aloys Engel (2005). Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_245. ISBN 978-3527306732.
- ↑ "Sigma-Aldrich, 60% Trypan Blue, Product page". http://www.sigmaaldrich.com/catalog/product/aldrich/302643?lang=en®ion=US.
- ↑ Strober, W (May 2001). Trypan Blue Exclusion Test of Cell Viability. Appendix 3. Appendix 3B. doi:10.1002/0471142735.ima03bs21. ISBN 978-0471142737.
- ↑ Nowicki, Marcin (15 May 2013), "A simple dual stain for detailed investigations of plant-fungal pathogen interactions", Vegetable Crops Research Bulletin 77: 61–74, doi:10.2478/v10032-012-0016-z
- ↑ "Trypan Blue Dye: Capsular Staining for Cataract Surgery and More" (in en). 1 April 2006. https://www.aao.org/current-insight/trypan-blue-dye-capsular-staining-cataract-surgery.
fr:Bleu de trypan#Coloration au bleu de trypan
 |