Chemistry:Violanthrone
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Anthra[10,1,2-cde]benzo[rst]pentaphene-5,10-dione | |
| Other names
Dibenzanthrone, Tinon Dark Blue BOA, Ahcovat Dark Blue BO, Violanthrone A, Bianthrone A, Irgalite Blue 2R, Paradone Dark Blue
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C34H16O2 | |
| Molar mass | 456.48964 |
| Appearance | dark blue solid |
| Density | 1.53 g/cm3 |
| -204.8·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
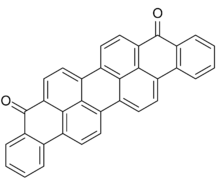
Violanthrone, also known as dibenzanthrone, is an organic compound that serves as a vat dye and a precursor to other vat dyes. X-ray crystallography confirms that the molecule is planar with C2v symmetry.[1] Isomeric with violanthrone is isoviolanthrone, which has a centrosymmetric structure.[2]
Synthesis
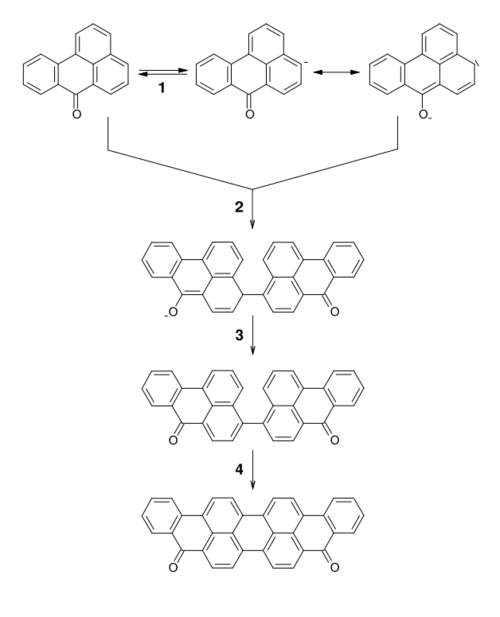
It is produced by coupling of two molecules of benzanthrone.[3][4][5][6]
References
- ↑ Bolton, W.; Stadler, H. P. (1964). "The Crystal Structure of Violanthrone (Dibenzanthrone)". Acta Crystallographica 17 (8): 1015–1020. doi:10.1107/S0365110X64002584.
- ↑ Bien, H.-S.; Stawitz, J.; Wunderlich, K.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
- ↑ Manufacture of dibenzanthrone compounds
- ↑ Heinrich Zollinger, Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd edition, Wiley-VCH, Weinheim, 2003, ISBN:3-906390-23-3, p. 291
- ↑ Wang Hongwei, Xu Huixiang, Li Zhen, Jiang Dawei, Gao Hongyu, Guo Yuan, "A kind of preparation method of Vat Brilliant Green FFB", CN patent 106243769
- ↑ Aoki, Junji (December 1961). "Studies of Violanthrone B. I. Reduction and Oxidation of Violanthrone B". Bulletin of the Chemical Society of Japan 34 (12): 1817–1819. doi:10.1246/bcsj.34.1817. https://www.journal.csj.jp/doi/pdf/10.1246/bcsj.34.1817. Retrieved 7 October 2021.
 |