Chemistry:Viridicatumtoxin B
| |
| Names | |
|---|---|
| IUPAC name
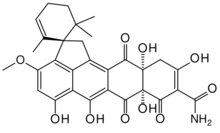
(1S,7a'S,11a'R)-5',6',7a',10',11a'-Pentahydroxy-3'-methoxy-2,6,6-trimethyl-7',8',12'-trioxo-7',7a',8',11',11a',12'-hexahydro-1'H-spiro[cyclohex-2-ene-1,2'-cyclopenta[de]tetracene]-9'-carboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| C30H29NO10 | |
| Molar mass | 563.559 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Viridicatumtoxin B is a fungus-derived tetracycline-like antibiotic discovered in 2008. It was isolated from small amounts of penicillium fungi. A synthetic structure matching that of natural viridicatumtoxin B makes possible synthetic variants that match or surpass its antibiotic potency.[1]
Analogs lacking a hydroxyl group were even more effective than the original against Gram-positive bacteria.[1]
Concerns about solubility, biodegradation, availability and other issues must be resolved before clinical development begins.[1]
History
The substance was first isolated from the mycelium of liquid fermentation cultures of Penicillium species FR11.[2]
Structure
Based on mass spectrometry and nuclear magnetic resonance data, the substance was originally thought to be the 11a',12'-epoxide,[2] but the structure was later revised.[3]
Effects
Viridicatumtoxin B inhibited the growth of Staphylococcus aureus, including methicillin resistant S. aureus and quinolone-resistant S. aureus with a minimum inhibitory concentration of 0.5 μg/ml. That effect is similar to that of vancomycin, but 8 to 64 times greater than that of tetracycline.[2]
Total synthesis
A complete total synthesis of viridicatumtoxin B, in racemic form, was completed in 2013 by the group of K. C. Nicolaou.[3][4]
See also
References
- ↑ 1.0 1.1 1.2 "Synthesis produces new antibiotic". 28 August 2014. http://www.rdmag.com/news/2014/08/synthesis-produces-new-antibiotic.
- ↑ 2.0 2.1 2.2 Zheng, C. J.; Yu, H. E.; Kim, E. H.; Kim, W. G. (2008). "Viridicatumtoxin B, a new anti-MRSA agent from Penicillium sp. FR11". The Journal of Antibiotics 61 (10): 633–7. doi:10.1038/ja.2008.84. PMID 19168978.
- ↑ 3.0 3.1 Nicolaou, K. C.; Hale, Christopher R. H.; Nilewski, Christian; Ioannidou, Heraklidia A.; Elmarrouni, Abdelatif; Nilewski, Lizanne G.; Beabout, Kathryn; Wang, Tim T. et al. (2014). "Total Synthesis of Viridicatumtoxin B and Analogues Thereof: Strategy Evolution, Structural Revision, and Biological Evaluation". Journal of the American Chemical Society 136 (34): 12137–60. doi:10.1021/ja506472u. PMID 25317739.
- ↑ Nicolaou, K. C.; Nilewski, Christian; Hale, Christopher R. H.; Ioannidou, Heraklidia A.; Elmarrouni, Abdelatif; Koch, Lizanne G. (2013). "Total Synthesis and Structural Revision of Viridicatumtoxin B". Angewandte Chemie International Edition 52 (33): 8736–41. doi:10.1002/anie.201304691. PMID 23893651.
 |

