Biology:1-Aminocyclopropane-1-carboxylate synthase
| 1-aminocyclopropane-1-carboxylate synthase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
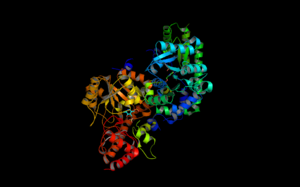
 Structure of ACC Synthase | |||||||||
| Identifiers | |||||||||
| EC number | 4.4.1.14 | ||||||||
| CAS number | 72506-68-4 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The enzyme aminocyclopropane-1-carboxylic acid synthase (ACC synthase, ACS) (EC 4.4.1.14) catalyzes the synthesis of 1-Aminocyclopropane-1-carboxylic acid (ACC), a precursor for ethylene, from S-Adenosyl methionine (AdoMet, SAM), an intermediate in the Yang cycle and activated methyl cycle and a useful molecule for methyl transfer:
- S-adenosyl-L-methionine = 1-aminocyclopropane-1-carboxylate + S-methyl-5′-thioadenosine
Like other PLP dependent enzymes, it catalyzes the reaction through a quinonoid zwitterion intermediate and uses cofactor pyridoxal phosphate (PLP, the active form of vitamin B6) for stabilization.[1][2][3]
This enzyme belongs to the family of lyases, specifically carbon-sulfur lyases. The systematic name of this enzyme class is S-adenosyl-L-methionine S-methyl-5′-thioadenosine-lyase (1-aminocyclopropane-1-carboxylate-forming). Other names in common use include 1-aminocyclopropanecarboxylate synthase, 1-aminocyclopropane-1-carboxylic acid synthase, 1-aminocyclopropane-1-carboxylate synthetase, aminocyclopropanecarboxylic acid synthase, aminocyclopropanecarboxylate synthase, ACC synthase, and S-adenosyl-L-methionine methylthioadenosine-lyase. This enzyme participates in propanoate metabolism. It employs one cofactor, pyridoxal phosphate.
Enzyme mechanism
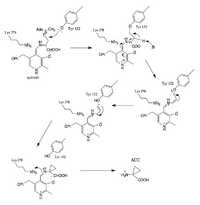
The reaction catalyzed by 1-aminocyclopropane-1-carboxylic acid synthase (ACS) is the committed and rate-limiting step in the biosynthesis of ethylene [20], a gaseous plant hormone that is responsible for the initiation of fruit ripening, shoot and root growth and differentiation, leaf and fruit abscission, flower opening, and flower and leaf senescence. (source) It is a pyridoxal phosphate (PLP) dependent gamma-elimination (?). In the gamma elimination, PLP acts as a sink twice (absorbing electrons from two deprotonations).[4][5]
Proposed steps of the reaction mechanism:
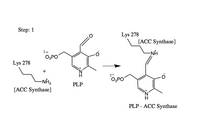
- Formation of the ACS-PLP Schiff Base
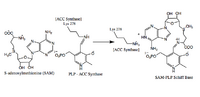
- Imine Exchange
- Formation of the Quinonoid Intermediate
- Tyrosine and PLP stabilized 3C-Ring formation
- Formation of the ACS-PLP Schiff Base
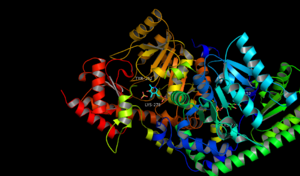
The aldehyde of coenzyme PLP reacts to form an imine (Schiff base) linkage with the catalytic domain lysine (278) residue of ACS.
- Imine exchange
An imine exchange occurs, and the amine nitrogen of the substrate, S-Adenosyl methionine, replaces Lys (278) in the imine linkage. (Stabilized by H bonding).
- Formation of the Quinonoid Intermediate
PLP acts as an 'electron sink' absorbing delocalized electron density during the reaction intermediates (countering the excess electron density on the deprotonated a-carbon). PLP facilitates the enzyme activity, increasing the acidity of the alpha carbon by stabilizing the conjugate base. The PLP-stabilized carbanion intermediate formed is the quinonoid intermediate.
- Tyrosine and PLP stabilized 3C-Ring formation
PLP and Tyrosine stabilize negative charges during deprotonation. Tyrosine attacks the sulfur bound carbon, allowing S(CH3)(Ado) to leave, and during ring formation, Tyrosine leaves.
Regulation
ACC synthase reaches optimal activity in conditions of pH 8.5 and with Km = 20 um relative to its substrate, SAM.
ACC Synthase and ethylene biosynthesis are regulated by a whole host of stimuli. Stresses such as wounding, noxious chemicals, auxin, flooding, and indole-3-acetic acid (IAA) promote ethylene synthesis, creating a positive feedback cycle with ACC synthase, up-regulating its activity.
However, it is also inhibited by a number of compounds as well. S-Adenosylethionine can bind as a substrate for ACC synthase (with higher affinity than SAM) and therefore inhibit any reaction with SAM. ACC Synthase is also competitively inhibited by aminoethoxyvinylglycine (AVG) and aminooxyacetic acid (AOA), inhibitors to many pyridoxal phosphate-mediated enzymic reactions. They are natural toxins that cause slow binding inhibition by interfering with the coenzyme pyridoxal phosphate. ACC synthase activity is also inhibited by intermediates of the activated methyl cycle and the methionine-recycling pathway: 5′-methylthioadenosine, α-keto-γ-methylthiobutyric acid, and S-adenosylhomocysteine.[8][9][10]
Structure
ACC Synthase is 450-516 amino acid long sequence depending on the species of plant from which it is extracted. Though it is comparable in the species in which it is found, its COOH terminal domain is more variable, leading to differences such as oligomerization. The COOH terminal domain is responsible for oligomerization. In most ACC Synthase producing cells, ACC Synthase exists as a dimer. However, in some we find a monomer ("which is more active and efficient [than its dimer counterpart").[11]
The structure of ACS has been largely determined via X-ray crystallography.[12] Conservation of the residues in ACS's catalytic domain and sequence homology suggest that ACS catalyzes the synthesis of ACC in a similar fashion as other enzymes that require PLP as a cofactor. However, unlike many other PLP-dependent enzymes, Lys (278) is not the only residue that interacts with the substrate. The proximity of the electronegative oxygen from Tyr (152) to the C-γ-S bond suggests a crucial role in the formation of ACC.[13] X-ray crystallography with aminoethoxyvinylglycine (AVG) a competitive inhibitor confirmed Tyrosine's role in the γ elimination.[14]
As of late 2007, 6 structures have been solved for this class of enzymes, with PDB accession codes 1B8G, 1IAX, 1IAY, 1M4N, 1M7Y, and 1YNU.
Catalytic domain
The main functional groups in the catalytic domains are the Nitrogen from the Lys 278 residue and the Oxygen from the Tyrosine 152 residue.
Biological function and applications
ACC Synthase is the key, rate limiting step in ethylene synthesis. Because the up-regulation of ACC-Synthase is what induces fruit ripening and often spoilage there is more research being done on the regulatory mechanisms and biosynthetic pathways of ethylene to avoid spoilage.[15][16]
Notes
- ↑ "Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase—the ethylene-forming enzyme". Chem. Biol. 11 (10): 1383–94. October 2004. doi:10.1016/j.chembiol.2004.08.012. PMID 15489165.
- ↑ "Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene". J. Mol. Biol. 294 (3): 745–56. December 1999. doi:10.1006/jmbi.1999.3255. PMID 10610793.
- ↑ "Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5'-phosphate provide new insight into catalytic mechanisms". J. Biol. Chem. 276 (41): 38210–6. October 2001. doi:10.1074/jbc.M103840200. PMID 11431475.
- ↑ "Tyr152 plays a central role in the catalysis of 1-aminocyclopropane-1-carboxylate synthase". J. Exp. Bot. 56 (418): 2203–10. August 2005. doi:10.1093/jxb/eri220. PMID 15983009.
- ↑ "Apple 1-aminocyclopropane-1-carboxylate synthase in complex with the inhibitor L-aminoethoxyvinylglycine. Evidence for a ketimine intermediate". J. Biol. Chem. 277 (51): 49735–42. December 2002. doi:10.1074/jbc.M208427200. PMID 12228256.
- ↑ "Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5'-phosphate provide new insight into catalytic mechanisms". J. Biol. Chem. 276 (41): 38210–6. October 2001. doi:10.1074/jbc.M103840200. PMID 11431475.
- ↑ "Ethylene biosynthesis and signaling networks". Plant Cell 14 Suppl: S131–51. 2002. doi:10.1105/tpc.001768. PMID 12045274.
- ↑ "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. March 1992. doi:10.1073/pnas.89.6.2475. PMID 1549612. Bibcode: 1992PNAS...89.2475Y.
- ↑ "Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase". Plant Physiol. 72 (1): 139–45. May 1983. doi:10.1104/pp.72.1.139. PMID 16662947.
- ↑ "Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers". Development 138 (16): 3485–95. August 2011. doi:10.1242/dev.065102. PMID 21771812.
- ↑ "Differential regulation of 1-aminocyclopropane-1-carboxylate synthase gene family and its role in phenotypic plasticity in Stellaria longipes". Plant Mol. Biol. 36 (2): 265–74. January 1998. doi:10.1023/A:1005994118535. PMID 9484438.
- ↑ "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. March 1992. doi:10.1073/pnas.89.6.2475. PMID 1549612. Bibcode: 1992PNAS...89.2475Y.
- ↑ "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. March 1992. doi:10.1073/pnas.89.6.2475. PMID 1549612. Bibcode: 1992PNAS...89.2475Y.
- ↑ "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. March 1992. doi:10.1073/pnas.89.6.2475. PMID 1549612. Bibcode: 1992PNAS...89.2475Y.
- ↑ "Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase". Plant Physiol. 72 (1): 139–45. May 1983. doi:10.1104/pp.72.1.139. PMID 16662947.
- ↑ "Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening". Plant Physiol. 118 (4): 1295–305. December 1998. doi:10.1104/pp.118.4.1295. PMID 9847103.
References
- Lin, E. C. C.; Kistler, W. S.; Zwaig, N. (June 1970). "Glycerol Kinase, the Pacemaker for the Dissimilation of Glycerol in Escherichia coli". Journal of Bacteriology 102 (3): 753–759. doi:10.1128/JB.102.3.753-759.1970. PMID 4914079.
- Nakatsuka, Akira; Murachi, Shiho; Okunishi, Hironori; Shiomi, Shinjiro; Nakano, Ryohei; Kubo, Yasutaka; Inaba, Akitsugu (1998). "Differential Expression and Internal Feedback Regulation of 1-Aminocyclopropane-1-Carboxylate Synthase, 1-Aminocyclopropane-1-Carboxylate Oxidase, and Ethylene Receptor Genes in Tomato Fruit during Development and Ripening". Plant Physiology 118 (4): 1295–1305. doi:10.1104/pp.118.4.1295. PMID 9847103.
- Wang, H.; Mei, W.; Qin, Y.; Zhu, Y. (2011). "1-Aminocyclopropane-1-carboxylic acid synthase 2 is phosphorylated by calcium-dependent protein kinase 1 during cotton fiber elongation". Acta Biochimica et Biophysica Sinica 43 (8): 654–661. doi:10.1093/abbs/gmr056. PMID 21742672.
- Wang, Long-Chi; Hsu, Jen-Hung; Lin, Lee-Chung (2010-10-22). "Identification of Novel Inhibitors of 1-Aminocyclopropane-1-carboxylic Acid Synthase by Chemical Screening in Arabidopsis thaliana". Journal of Biological Chemistry 285 (43): 33445–33456. doi:10.1074/jbc.M110.132498. PMID 20682786.
- "Ethylene Biosynthesis and Signaling Networks". http://www.acrbuynow.info/content/14/suppl_1/S131.full+.
- Guido Capitani; Markus Tschopp. "Structure of ACC synthase inactivated by the mechanism-based inhibitor L-vinylglycine". www.bioc.uzh.ch. http://www.bioc.uzh.ch/gruetter/publications_files/2005/capitani_b.pdf.
- "jxb.oxfordjournals.org". http://jxb.oxfordjournals.org/content/56/418/2203.full.pdf.
- Li, Jian-Feng; Qu, Liang-Hu; Li, Ning (2005). "jxb.oxfordjournals.org". Journal of Experimental Botany 56 (418): 2203–2210. doi:10.1093/jxb/eri220. PMID 15983009.
- "Crystal structure of highly thermostable glycerol kinase from a hyperthermophilic archaeon in a dimeric form". FEBS J. 275 (10): 2632–43. May 2008. doi:10.1111/j.1742-4658.2008.06410.x. PMID 18422647.
- "Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening". Plant Physiol. 118 (4): 1295–305. December 1998. doi:10.1104/pp.118.4.1295. PMID 9847103.
- "1-Aminocyclopropane-1-carboxylic acid synthase 2 is phosphorylated by calcium-dependent protein kinase 1 during cotton fiber elongation". Acta Biochim. Biophys. Sin. (Shanghai) 43 (8): 654–61. August 2011. doi:10.1093/abbs/gmr056. PMID 21742672.
- "Structure of ACC synthase inactivated by the mechanism-based inhibitor L-vinylglycine". FEBS Lett. 579 (11): 2458–62. April 2005. doi:10.1016/j.febslet.2005.03.048. PMID 15848188.
- "Tyr152 plays a central role in the catalysis of 1-aminocyclopropane-1-carboxylate synthase". J. Exp. Bot. 56 (418): 2203–10. August 2005. doi:10.1093/jxb/eri220. PMID 15983009.
- "Apple 1-aminocyclopropane-1-carboxylate synthase in complex with the inhibitor L-aminoethoxyvinylglycine. Evidence for a ketimine intermediate". J. Biol. Chem. 277 (51): 49735–42. December 2002. doi:10.1074/jbc.M208427200. PMID 12228256.
- "Crystal structures of 1-aminocyclopropane-1-carboxylate (ACC) synthase in complex with aminoethoxyvinylglycine and pyridoxal-5'-phosphate provide new insight into catalytic mechanisms". J. Biol. Chem. 276 (41): 38210–6. October 2001. doi:10.1074/jbc.M103840200. PMID 11431475.
- "The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato". Plant Physiol. 123 (3): 979–86. July 2000. doi:10.1104/pp.123.3.979. PMID 10889246.
- "Crystal structure and mechanistic implications of 1-aminocyclopropane-1-carboxylic acid oxidase--the ethylene-forming enzyme". Chem. Biol. 11 (10): 1383–94. October 2004. doi:10.1016/j.chembiol.2004.08.012. PMID 15489165.
- "Structure of 1-aminocyclopropane-1-carboxylate synthase, a key enzyme in the biosynthesis of the plant hormone ethylene". J. Mol. Biol. 294 (3): 745–56. December 1999. doi:10.1006/jmbi.1999.3255. PMID 10610793.
- Holland RR (August 1975). "Decision tables. Their use for the presentation of clinical algorithms". JAMA 233 (5): 455–7. doi:10.1001/jama.1975.03260050061028. PMID 1080212.
- "Differential regulation of 1-aminocyclopropane-1-carboxylate synthase gene family and its role in phenotypic plasticity in Stellaria longipes". Plant Mol. Biol. 36 (2): 265–74. January 1998. doi:10.1023/A:1005994118535. PMID 9484438.
- "Differential accumulation of transcripts for four tomato 1-aminocyclopropane-1-carboxylate synthase homologs under various conditions". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2475–9. March 1992. doi:10.1073/pnas.89.6.2475. PMID 1549612. Bibcode: 1992PNAS...89.2475Y.
- "Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase". Plant Physiol. 72 (1): 139–45. May 1983. doi:10.1104/pp.72.1.139. PMID 16662947.
- "Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers". Development 138 (16): 3485–95. August 2011. doi:10.1242/dev.065102. PMID 21771812.
- "Slow-binding inhibition of Escherichia coli cystathionine beta-lyase by L-aminoethoxyvinylglycine: a kinetic and X-ray study". Biochemistry 36 (41): 12633–43. October 1997. doi:10.1021/bi970630m. PMID 9376370.
- "Ethylene biosynthesis and signaling networks". Plant Cell 14 Suppl: S131–51. 2002. doi:10.1105/tpc.001768. PMID 12045274.
- "Assay for and enzymatic formation of an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid". Planta 145 (3): 293–303. 1979. doi:10.1007/BF00454455. PMID 24317737.
- "1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis". Arch. Biochem. Biophys. 198 (1): 280–6. 1979. doi:10.1016/0003-9861(79)90420-X. PMID 507845.
 |