Chemistry:S-Adenosyl methionine
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
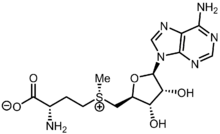
(2S)-2-Amino-4-[(S)-{[(2S,3S,4R,5R)-5-(4-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}methylsulfaniumyl]butanoate | |||
| Other names
S-Adenosyl-L-methionine; SAM-e; SAMe, AdoMet, Heparab (India), ademethionine
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | S-Adenosylmethionine | ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C15H22N6O5S | |||
| Molar mass | 398.44 g·mol−1 | ||
| Pharmacology | |||
| 1=ATC code }} | A16AA02 (WHO) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
S-Adenosyl methionine (SAM-e) is a common cosubstrate involved in methyl group transfers, transsulfuration, and aminopropylation. Although these anabolic reactions occur throughout the body, most SAM-e is produced and consumed in the liver.[1] More than 40 methyl transfers from SAM-e are known, to various substrates such as nucleic acids, proteins, lipids and secondary metabolites. It is made from adenosine triphosphate (ATP) and methionine by methionine adenosyltransferase. SAM-e was first discovered by Giulio Cantoni in 1952.[1]
In bacteria, SAM-e is bound by the SAM riboswitch, which regulates genes involved in methionine or cysteine biosynthesis. In eukaryotic cells, SAM-e serves as a regulator of a variety of processes including DNA, tRNA, and rRNA methylation; immune response;[2] amino acid metabolism; transsulfuration; and more. In plants, SAM-e is crucial to the biosynthesis of ethylene, an important plant hormone and signaling molecule.[3]
Chemically, it is a sulfonium betaine which serves as a source of electrophilic methyl group or as a source of 5'-deoxyadenosyl radical.
Biochemistry
SAM-e cycle
The reactions that produce, consume, and regenerate SAM-e are called the SAM-e cycle. In the first step of this cycle, the SAM-dependent methylases (EC 2.1.1) that use SAM-e as a substrate produce S-adenosyl homocysteine as a product.[4] S-Adenosyl homocysteine is a strong negative regulator of nearly all SAM-dependent methylases despite their biological diversity. This is hydrolysed to homocysteine and adenosine by S-adenosylhomocysteine hydrolase EC 3.3.1.1 and the homocysteine recycled back to methionine through transfer of a methyl group from 5-methyltetrahydrofolate, by one of the two classes of methionine synthases (i.e. cobalamin-dependent (EC 2.1.1.13) or cobalamin-independent (EC 2.1.1.14)). This methionine can then be converted back to SAM-e, completing the cycle.[5] In the rate-limiting step of the SAM cycle, MTHFR (methylenetetrahydrofolate reductase) irreversibly reduces 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate.[6]
Radical SAM-e enzymes
A large number of iron-sulfur cluster-containing enzymes cleave SAM-e reductively to produce a 5′-deoxyadenosyl 5′-radical as an intermediate, and are called radical SAM enzymes.[7] Most enzymes with this capability share a region of sequence homology that includes the motif CxxxCxxC or a close variant. The radical intermediate allows enzymes to perform a wide variety of unusual chemical reactions. Examples of radical SAM enzymes include spore photoproduct lyase, activases of pyruvate formate lyase and anaerobic sulfatases, lysine 2,3-aminomutase, and various enzymes of cofactor biosynthesis, peptide modification, metalloprotein cluster formation, tRNA modification, lipid metabolism, etc. Some radical SAM-e enzymes use a second SAM-e as a methyl donor. Radical SAM enzymes are much more abundant in anaerobic bacteria than in aerobic organisms. They can be found in all domains of life and are largely unexplored. A recent bioinformatics study concluded that this family of enzymes includes at least 114,000 sequences including 65 unique reactions.[8]
Polyamine biosynthesis
Another major role of SAM-e is in polyamine biosynthesis. Here, SAM-e is decarboxylated by adenosylmethionine decarboxylase (EC 4.1.1.50) to form S-adenosylmethioninamine. This compound then donates its n-propylamine group in the biosynthesis of polyamines such as spermidine and spermine from putrescine.[9]
SAM-e is required for cellular growth and repair. It is also involved in the biosynthesis of several hormones and neurotransmitters that affect mood, such as epinephrine. Methyltransferases are also responsible for the addition of methyl groups to the 2′ hydroxyls of the first and second nucleotides next to the 5′ cap in messenger RNA.[10][11]
Therapeutic uses
As of 2012 the evidence was inconclusive as to whether SAM can mitigate the pain of osteoarthritis; clinical trials that had been conducted were too small from which to generalize.[12]
The SAM-e cycle has been closely tied to the liver since 1947 because people with alcoholic cirrhosis of the liver would accumulate large amounts of methionine in their blood.[13] While multiple lines of evidence from laboratory tests on cells and animal models suggest that SAM might be useful to treat various liver diseases, as of 2012 SAM had not been studied in any large randomized placebo-controlled clinical trials that would allow an assessment of its efficacy and safety.[14][15]
Depression
A 2016 Cochrane review concluded that for major depressive disorder, "Given the absence of high quality evidence and the inability to draw firm conclusions based on that evidence, the use of SAMe for the treatment of depression in adults should be investigated further."[16]
A 2020 systematic review found no statistically different outcome comparing SAMe and other commonly used antidepressant (imipramine or escitalopram).[17]
Pharmacokinetics
Oral SAM achieves peak plasma concentrations three to five hours after ingestion of an enteric-coated tablet (400–1000 mg). The half-life is about 100 minutes.[18]
Adverse effects
Gastrointestinal disorder, dyspepsia and anxiety can occur with SAM consumption.[18] Long-term effects are unknown. SAM is a weak DNA-alkylating agent.[19]
Another reported side effect of SAM is insomnia; therefore, the supplement is often taken in the morning. Other reports of mild side effects include lack of appetite, constipation, nausea, dry mouth, sweating, and anxiety/nervousness, but in placebo-controlled studies, these side effects occur at about the same incidence in the placebo groups.
SAM-e has recently been shown to play a role in epigenetic regulation. DNA methylation is a key regulator in epigenetic modification during mammalian cell development and differentiation. In mouse models, excess levels of SAM-e have been implicated in erroneous methylation patterns associated with diabetic neuropathy. SAM-e serves as the methyl donor in cytosine methylation, which is a key epigenetic regulatory process.[20] Because of this impact on epigenetic regulation, SAM-e has been tested as an anti-cancer treatment. Cancer cell proliferation is dependent on having low levels of DNA methylation. In vitro addition has been shown to remethylate promoter sequences and decrease the production of proto-oncogenes.[21]
Deficiencies in radical SAM-e enzymes have been associated with a variety of diseases including congenital heart disease, amyotrophic lateral sclerosis, and increased viral susceptibility.[8]
Interactions and contraindications
Taking SAM at the same time as some drugs may increase the risk of serotonin syndrome, a potentially dangerous condition caused by having too much serotonin. These drugs include dextromethorphan (Robitussin), meperidine (Demerol), pentazocine (Talwin), and tramadol (Ultram). SAM may also interact with antidepressant medications increasing the potential for their side effects and reduce the effectiveness of levodopa for Parkinson's disease.
People who have bipolar disorder should not use SAM because it increases the risk of manic episodes.[22]
Availability in different countries
In the United States and Canada , SAM is sold as a dietary supplement under the marketing name SAM-e (also spelled SAME or SAMe; pronounced "Sammy";[23]) it was introduced in the US in 1999, after the Dietary Supplement Health and Education Act was passed in 1994.[24]
It was introduced as a prescription drug in Italy in 1979, in Spain in 1985, and in Germany in 1989;[24] as of 2012, it was marketed as a prescription drug in Russia, India, China, Italy, Germany, Vietnam, and Mexico.[15]
See also
- DNA methyltransferase
- SAM-I riboswitch
- SAM-II riboswitch
- SAM-III riboswitch
- SAM-IV riboswitch
- SAM-V riboswitch
- SAM-VI riboswitch
- List of investigational antidepressants
References
- ↑ 1.0 1.1 Cantoni, GL (1952). "The Nature of the Active Methyl Donor Formed Enzymatically from L-Methionine and Adenosinetriphosphate". J Am Chem Soc 74 (11): 2942–3. doi:10.1021/ja01131a519.
- ↑ Ding, Wei; Smulan, Lorissa J.; Hou, Nicole S.; Taubert, Stefan; Watts, Jennifer L.; Walker, Amy K. (2015-10-06). "S-Adenosylmethionine Levels Govern Innate Immunity through Distinct Methylation-Dependent Pathways". Cell Metabolism 22 (4): 633–645. doi:10.1016/j.cmet.2015.07.013. PMID 26321661.
- ↑ Wang, X.; Oh, M. W.; Komatsu, S. (2016-06-01). "Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses" (in en). Biologia Plantarum 60 (2): 269–278. doi:10.1007/s10535-016-0586-6. ISSN 0006-3134.
- ↑ "Homocysteine". Int J Biochem Cell Biol 32 (4): 385–9. 2000. doi:10.1016/S1357-2725(99)00138-7. PMID 10762063.
- ↑ "Molecular biology of 5,10-methylenetetrahydrofolate reductase". J Nephrol 13 (1): 20–33. Jan–Feb 2000. PMID 10720211.
- ↑ Goyette, P.; Sumner, J. S.; Milos, R.; Duncan, A. M.; Rosenblatt, D. S.; Matthews, R. G.; Rozen, R. (1994-06-01). "Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification". Nature Genetics 7 (2): 195–200. doi:10.1038/ng0694-195. ISSN 1061-4036. PMID 7920641.
- ↑ Booker, SJ; Grove, TL (2010). "Mechanistic and functional versatility of radical SAM enzymes". F1000 Biology Reports 2: 52. doi:10.3410/B2-52. PMID 21152342.
- ↑ 8.0 8.1 Landgraf, Bradley J.; McCarthy, Erin L.; Booker, Squire J. (2016-06-13). "Radical S-Adenosylmethionine Enzymes in Human Health and Disease" (in en). Annual Review of Biochemistry 85: 485–514. doi:10.1146/annurev-biochem-060713-035504. PMID 27145839.
- ↑ Roje S (2006). "S-Adenosyl-L-methionine: beyond the universal methyl group donor". Phytochemistry 67 (15): 1686–98. doi:10.1016/j.phytochem.2006.04.019. PMID 16766004.
- ↑ Loenen W (2006). "S-Adenosylmethionine: jack of all trades and master of everything?". Biochem Soc Trans 34 (Pt 2): 330–3. doi:10.1042/BST20060330. PMID 16545107.
- ↑ "S-Adenosylmethionine and methylation". FASEB J 10 (4): 471–80. 1996. doi:10.1096/fasebj.10.4.8647346. PMID 8647346.
- ↑ Rutjes, AW; Nüesch, E; Reichenbach, S; Jüni, P (7 October 2009). "S-Adenosylmethionine for osteoarthritis of the knee or hip.". The Cochrane Database of Systematic Reviews (4): CD007321. doi:10.1002/14651858.CD007321.pub2. PMID 19821403. PMC 7061276. https://boris.unibe.ch/30335/1/Rutjes%20CochraneDatabaseSystRev%202009_CD007321.pdf.
- ↑ Mato, Jose M (1997). "S-adenosylmethionine synthesis: Molecular mechanisms and clinical implications". Pharmacology & Therapeutics 73 (3): 265–280. doi:10.1016/s0163-7258(96)00197-0. PMID 9175157.
- ↑ Anstee, QM; Day, CP (November 2012). "S-Adenosylmethionine (SAMe) therapy in liver disease: a review of current evidence and clinical utility". Journal of Hepatology 57 (5): 1097–109. doi:10.1016/j.jhep.2012.04.041. PMID 22659519. http://www.journal-of-hepatology.eu/article/S0168-8278(12)00409-6/pdf.
- ↑ 15.0 15.1 Lu, SC; Mato, JM (October 2012). "S-Adenosylmethionine in liver health, injury, and cancer". Physiological Reviews 92 (4): 1515–42. doi:10.1152/physrev.00047.2011. PMID 23073625.
- ↑ Galizia, I; Oldani, L; Macritchie, K; Amari, E; Dougall, D; Jones, TN; Lam, RW; Massei, GJ et al. (10 October 2016). "S-Adenosyl methionine (SAMe) for depression in adults.". The Cochrane Database of Systematic Reviews 10: CD011286. doi:10.1002/14651858.CD011286.pub2. PMID 27727432.
- ↑ Cuomo, Alessandro; Beccarini Crescenzi, Bruno; Bolognesi, Simone; Goracci, Arianna; Koukouna, Despoina; Rossi, Rodolfo; Fagiolini, Andrea (2020-09-05). "S-Adenosylmethionine (SAMe) in major depressive disorder (MDD): a clinician-oriented systematic review". Annals of general psychiatry (Springer Science and Business Media LLC) 19 (1). doi:10.1186/s12991-020-00298-z. ISSN 1744-859X. PMID 32939220.
- ↑ 18.0 18.1 "S-Adenosyl methionine (SAMe) versus celecoxib for the treatment of osteoarthritis symptoms: A double-blind cross-over trial. ISRCTN36233495". BMC Musculoskelet Disord 5: 6. February 2004. doi:10.1186/1471-2474-5-6. PMID 15102339.
- ↑ "Nonenzymatic methylation of DNA by the intracellular methyl group donor S-adenosyl-L-methionine is a potentially mutagenic reaction". EMBO J. 1 (2): 211–6. 1982. doi:10.1002/j.1460-2075.1982.tb01149.x. PMID 7188181.
- ↑ Varela-Rey, Marta (2014). "S-Adenosylmethionine Levels Regulate the Schwann Cell DNA Methylome". Neuron 81 (5): 1024–1039. doi:10.1016/j.neuron.2014.01.037. PMID 24607226.
- ↑ Schmidt, Thomas; Leha, Andreas; Salinas-Riester, Gabriela (2016-12-31). "Treatment of prostate cancer cells with S-adenosylmethionine leads to genome-wide alterations in transcription profiles". Gene 595 (2): 161–167. doi:10.1016/j.gene.2016.09.032. PMID 27688072.
- ↑ "S-Adenosyl-L-Methionine (SAMe): In Depth" (in en). NCCIH. January 11, 2017. https://nccih.nih.gov/health/supplements/SAMe.
- ↑ Woolston, Chris. "What is SAM-e?" 1 January 2019. Retrieved on 14 November 2019.
- ↑ 24.0 24.1 Bottiglieri, T (November 2002). "S-Adenosyl-L-methionine (SAMe): from the bench to the bedside--molecular basis of a pleiotrophic molecule". The American Journal of Clinical Nutrition 76 (5): 1151S–1157S. doi:10.1093/ajcn/76.5.1151S. PMID 12418493.
External links
- EINECS number 249-946-8
- Shippy, R Andrew; Mendez, Douglas; Jones, Kristina; Cergnul, Irene; Karpiak, Stephen E (2004). "S-Adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS". BMC Psychiatry 4: 38. doi:10.1186/1471-244X-4-38. PMID 15538952.
 |


