Biology:Arthropod defensin
Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro.[2][3][4][5][6] However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies.[7] It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence.[7]
Structure
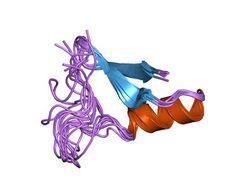
Arthropod defensin peptides range in length from 38 to 51 amino acids. There are six conserved cysteines all involved in intrachain disulfide bonds. Studies have shown that the cysteine-bridge disulfide bonds are not required for antimicrobial activity,[8] similar to findings in mammalian defensins.[9] Furthermore, it was also shown that the N-terminal helix region in arthropod or insect defensins is also not required for antimicrobial activity of these peptides.[8]
A schematic representation of peptides from the arthropod defensin family is shown below.
+----------------------------+
| |
| | | |
+---|---------------+ |
+-----------------+
'C': conserved cysteine involved in a disulfide bond.
Relation to other defensins
Sequence similarities have been reported between the arthropod defensins and mammalian defensins.[10][2] However it appears that defensins of vertebrates, arthropods, plants, and fungi arose independently.[11] This is supported by 3D structural differences between arthropod defensins and vertebrate beta defensins.[12] However structural similarities exist between these defensins, notably in two structural motifs termed "C6" and "C8". This has prompted a higher "cis-" or "tras-" defensin classification system wherein the structural relationships of the shared motifs is used to delineate defensin similarities.[11]
Activity against non-microbial cells
Defensins of mammals display anti-cancer activities in vitro,[13] and down-regulation of human beta-defensin 1 is associated with increased risk of prostate cancer and clear-cell carcinomas.[14] The first in vivo anti-cancer functions for defensin came from Drosophila studies, which showed that the Drosophila defensin attacks tumor cells, and that flies lacking defensin had greater tumor growth in a cancer disease model.[15][16]
Overactive immune signalling is also implicated in age-associated neurodegeneration,[17] and overexpression of defensin leads to increased degradation of brain tissue.[18]
Notes
- ↑ "Refined three-dimensional solution structure of insect defensin A". Structure 3 (5): 435–48. May 1995. doi:10.1016/S0969-2126(01)00177-0. PMID 7663941.
- ↑ Jump up to: 2.0 2.1 "Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides". Proceedings of the National Academy of Sciences of the United States of America 86 (1): 262–6. January 1989. doi:10.1073/pnas.86.1.262. PMID 2911573. Bibcode: 1989PNAS...86..262L.
- ↑ "A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin". The Journal of Biological Chemistry 265 (19): 11333–7. July 1990. doi:10.1016/S0021-9258(19)38596-5. PMID 2358464.
- ↑ "Purification, sequence and antibacterial activity of two novel sapecin homologues from Sarcophaga embryonic cells: similarity of sapecin B to charybdotoxin". The Biochemical Journal 291 ( Pt 1) (Pt 1): 275–9. April 1993. doi:10.1042/bj2910275. PMID 8471044.
- ↑ "Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family". The Journal of Biological Chemistry 266 (36): 24520–5. December 1991. doi:10.1016/S0021-9258(18)54260-5. PMID 1761552.
- ↑ "A novel insect defensin mediates the inducible antibacterial activity in larvae of the dragonfly Aeschna cyanea (Paleoptera, Odonata)". European Journal of Biochemistry 209 (3): 977–84. November 1992. doi:10.1111/j.1432-1033.1992.tb17371.x. PMID 1425705.
- ↑ Jump up to: 7.0 7.1 "Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach". eLife 8. February 2019. doi:10.7554/eLife.44341. PMID 30803481.
- ↑ Jump up to: 8.0 8.1 "Antibacterial activity of linear peptides spanning the carboxy-terminal beta-sheet domain of arthropod defensins". Peptides 27 (11): 2614–23. November 2006. doi:10.1016/j.peptides.2006.06.010. PMID 16914230.
- ↑ "Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines". Antimicrobial Agents and Chemotherapy 49 (11): 4561–6. November 2005. doi:10.1128/AAC.49.11.4561-4566.2005. PMID 16251296.
- ↑ "Big defensins, a diverse family of antimicrobial peptides that follows different patterns of expression in hemocytes of the oyster Crassostrea gigas". PLOS ONE 6 (9): e25594. 2011. doi:10.1371/journal.pone.0025594. PMID 21980497. Bibcode: 2011PLoSO...625594R.
- ↑ Jump up to: 11.0 11.1 "The Defensins Consist of Two Independent, Convergent Protein Superfamilies". Molecular Biology and Evolution 33 (9): 2345–56. September 2016. doi:10.1093/molbev/msw106. PMID 27297472.
- ↑ "1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin". FEBS Letters 269 (2): 413–20. September 1990. doi:10.1016/0014-5793(90)81206-4. PMID 2401368.
- ↑ "Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications". Oncotarget 8 (28): 46635–46651. July 2017. doi:10.18632/oncotarget.16743. PMID 28422728.
- ↑ "Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas". Laboratory Investigation; A Journal of Technical Methods and Pathology 83 (4): 501–5. April 2003. doi:10.1097/01.LAB.0000063929.61760.F6. PMID 12695553.
- ↑ "The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila.". eLife 8: e45061. July 2019. doi:10.7554/eLife.45061. PMID 31358113.
- ↑ "Acute respiratory infection in childhood: a study of parental prescribing patterns and advice sources". The New Zealand Medical Journal 96 (734): 481–2. June 1983. PMID 6602314.
- ↑ "NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration". Cell Reports 19 (4): 836–848. April 2017. doi:10.1016/j.celrep.2017.04.007. PMID 28445733.
- ↑ "Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain". Proceedings of the National Academy of Sciences of the United States of America 110 (19): E1752–60. May 2013. doi:10.1073/pnas.1306220110. PMID 23613578. Bibcode: 2013PNAS..110E1752C.
Further reading
- "Refined three-dimensional solution structure of insect defensin A". Structure 3 (5): 435–48. May 1995. doi:10.1016/S0969-2126(01)00177-0. PMID 7663941.
References
 |