Chemistry:Drosomycin
| Drosomycin | |||||||
|---|---|---|---|---|---|---|---|
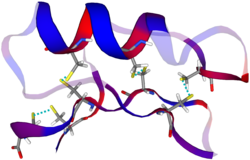 Model of the AMP drosomycin | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | Drs | ||||||
| UniProt | P41964 | ||||||
| |||||||
Drosomycin is an antifungal peptide from Drosophila melanogaster and was the first antifungal peptide isolated from insects.[1] Drosomycin is induced by infection by the Toll signalling pathway,[2] while expression in surface epithelia like the respiratory tract is instead controlled by the immune deficiency pathway (Imd).[3] This means that drosomycin, alongside other antimicrobial peptides (AMPs) such as cecropins,[4][5] diptericin,[6] drosocin,[7] metchnikowin[8] and attacin,[9] serves as a first line defence upon septic injury. However drosomycin is also expressed constitutively to a lesser extent in different tissues and throughout development.[10]
Structure
Drosomycin is a 44-residue defensin-like peptide containing four disulfide bridges. These bridges stabilize a structure involving one α-helix and three β-sheets. Owing to these four disulfide bridges, drosomycin is resistant to degradation and the action of proteases.[1][11][12] The cysteine stabilized αβ motif of drosomycin is also found in Drosophila defensin, and some plant defensins. Drosomycin has greater sequence similarity with these plant defensins (up to 40%), than with other insect defensins.[13] The structure was discovered in 1997 by Landon and his colleagues[14] The αβ motif of drosomycin is also found in a scorpion neurotoxin, and drosomycin potentiates the action of this neurotoxin on nerve excitation.[15]
Drosomycin multigene family
At the nucleotide level, drosomycin is a 387 bp-long gene (Drs) which lies on Muller element 3L,[16] very near six other drosomycin-like (Drsl) genes. These various drosomycins are referred to as the drosomycin multigene family. However, only drosomycin itself is part of the systemic immune response, while the other genes are regulated in different fashions. The antimicrobial activity of these various drosomycin-like peptides also differs.[17] In 2015 Gao and Zhu[18] found that in some Drosophila species (D. takahashii) some of these genes have been duplicated and this Drosophila has 11 genes in the drosomycin multigene family in total.
Function
It seems that drosomycin has about three major functions on fungi, the first is partial lysis of hyphae, the second is inhibition of spore germination (in higher concentrations of drosomycin), and the last is delaying of hypha growth, which leads to hyphae branching (at lower concentrations of drosomycin).[19] The exact mechanism of function to fungi still has to be clarified. In 2019, Hanson and colleagues[20] generated the first drosomycin mutant, finding that indeed flies lacking drosomycin were more susceptible to fungal infection.
References
- ↑ 1.0 1.1 "Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides". The Journal of Biological Chemistry 269 (52): 33159–63. December 1994. doi:10.1016/S0021-9258(20)30111-3. PMID 7806546.
- ↑ "The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults". Cell 86 (6): 973–83. September 1996. doi:10.1016/S0092-8674(00)80172-5. PMID 8808632. http://infoscience.epfl.ch/record/151754.
- ↑ "Drosomycin, an essential component of antifungal defence in Drosophila". Insect Molecular Biology 18 (5): 549–56. October 2009. doi:10.1111/j.1365-2583.2009.00907.x. PMID 19754735.
- ↑ "The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection". The EMBO Journal 9 (1): 217–24. January 1990. doi:10.1002/j.1460-2075.1990.tb08098.x. PMID 2104802.
- ↑ "CecC, a cecropin gene expressed during metamorphosis in Drosophila pupae". European Journal of Biochemistry 204 (1): 395–9. February 1992. doi:10.1111/j.1432-1033.1992.tb16648.x. PMID 1740152.
- ↑ "Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides". The Journal of Biological Chemistry 265 (36): 22493–8. December 1990. doi:10.1016/S0021-9258(18)45732-8. PMID 2125051.
- ↑ "A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution". The Journal of Biological Chemistry 268 (20): 14893–7. July 1993. doi:10.1016/S0021-9258(18)82417-6. PMID 8325867.
- ↑ "Metchnikowin, a novel immune-inducible proline-rich peptide from Drosophila with antibacterial and antifungal properties". European Journal of Biochemistry 233 (2): 694–700. October 1995. doi:10.1111/j.1432-1033.1995.694_2.x. PMID 7588819.
- ↑ "Identification of early genes in the Drosophila immune response by PCR-based differential display: the Attacin A gene and the evolution of attacin-like proteins". Insect Biochemistry and Molecular Biology 25 (4): 511–8. April 1995. doi:10.1016/0965-1748(94)00091-C. PMID 7742836. Bibcode: 1995IBMB...25..511A.
- ↑ "A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway". The EMBO Journal 17 (5): 1217–27. August 1998. doi:10.1093/emboj/17.5.1217. PMID 9482719.
- ↑ "Determination of the disulfide array of the first inducible antifungal peptide from insects: drosomycin from Drosophila melanogaster". FEBS Letters 395 (1): 6–10. October 1996. doi:10.1016/0014-5793(96)00992-1. PMID 8849679. Bibcode: 1996FEBSL.395....6M.
- ↑ "Differential display of peptides induced during the immune response of Drosophila: a matrix-assisted laser desorption ionization time-of-flight mass spectrometry study". Proceedings of the National Academy of Sciences of the United States of America 95 (19): 11342–7. September 1998. doi:10.1073/pnas.95.19.11342. PMID 9736738. Bibcode: 1998PNAS...9511342U.
- ↑ "Determination of the three-dimensional solution structure of Raphanus sativus antifungal protein 1 by 1H NMR". Journal of Molecular Biology 279 (1): 257–70. May 1998. doi:10.1006/jmbi.1998.1767. PMID 9636715.
- ↑ "Solution structure of drosomycin, the first inducible antifungal protein from insects". Protein Science 6 (9): 1878–84. September 1997. doi:10.1002/pro.5560060908. PMID 9300487.
- ↑ "Drosomycin, an innate immunity peptide of Drosophila melanogaster, interacts with the fly voltage-gated sodium channel". The Journal of Biological Chemistry 284 (35): 23558–63. August 2009. doi:10.1074/jbc.M109.023358. PMID 19574227.
- ↑ "Drs Drosomycin [Drosophila melanogaster (fruit fly) – Gene – NCBI"]. https://www.ncbi.nlm.nih.gov/gene/38419.
- ↑ "The evolution of antifungal peptides in Drosophila". Genetics 171 (4): 1847–59. December 2005. doi:10.1534/genetics.105.045435. PMID 16157672.
- ↑ "The drosomycin multigene family: three-disulfide variants from Drosophila takahashii possess antibacterial activity". Scientific Reports 6. August 2016. doi:10.1038/srep32175. PMID 27562645. Bibcode: 2016NatSR...632175G.
- ↑ "Antimicrobial peptides in insects; structure and function". Developmental and Comparative Immunology 23 (4–5): 329–44. 1999-06-01. doi:10.1016/S0145-305X(99)00015-4. PMID 10426426.
- ↑ "Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach". eLife 8. February 2019. doi:10.7554/eLife.44341. PMID 30803481.
 |
