Biology:CARD-CC family
The CARD-CC protein family is defined by an evolutionary conserved "caspase activation and recruitment domain" (CARD) and a coiled-coil (CC) domain.[1][2] Coiled-coils (CC) act as oligomerization domains for many proteins such as structural and motor proteins, and transcription factors. This means that monomers are converted to macromolecular complexes by polymerization.[3] In humans and other jawed vertebrates, the family consists of CARD9 and the three "CARD-containing MAGUK protein" (CARMA)[4] proteins CARD11 (CARMA1), CARD14 (CARMA2) and CARD10 (CARMA3). Although the MAGUK protein DLG5 contains both a CARD domain and a CC domain, it does not belong to the same family as the CARD-CC proteins since the evolutionary origin of its CARD domain is very likely to be different.[5]
Evolution and species distribution
The protein family is ancient and can be found as far back as Cnidaria, but has almost exclusively been studied in humans and mice. Notably, the protein family is absent in insects and nematodes, which makes it impossible to study its function in the most popular invertebrate model organisms (Drosophila and C. elegans). Invertebrates only have a CARD9-like ancestral CARD-CC member, and the earliest occurrence of a CARD-CC member with the CARMA domain composition is in the jawless vertebrate hagfish. Already in sharks are all four CARD-CC family members present, indicating that the 3 distinct CARMA CARD-CC family members were formed by two duplication events just before or very early in the jawed vertebrate evolution, almost half a billion years ago. The four CARD-CC ohnologous members in mice and humans differ in expression domains, where CARD9 is mostly expressed in myelocytes, CARD11 in lymphocytes, while CARD10 and CARD14 are mostly expressed in non-haematopoetic cells. This gene expression differentiation between the four CARD-CC family members conserved at least as far back as frogs (Xenopus tropicalis) and fish (Danio rerio),[6] indicating that the four CARD-CC family members have had distinct functions since early jawed vertebrate evolution.
Functions
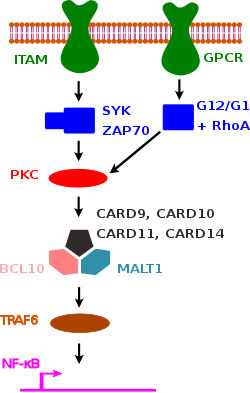
A common theme for all four CARD-CC family proteins in mice and humans is that they are activated by different protein kinase C isoforms,[7] and recruit BCL10 and the paracaspase MALT1 upon activation, forming a so-called CBM complex. There are four different CBM complexes, defined by which CARD-CC family member that is responsible for its assembly: CBM-9 (CARD9), CBM-1 (CARD11/CARMA1), CBM-2 (CARD14/CARMA2) and CBM-3 (CARD10/CARMA3).[8] CBM complex assembly results in recruitment of TRAF6 to MALT1 and downstream activation of NF-κB transcriptional activity and expression of pro-inflammatory cytokines. The different CARD-CC family members show different expression pattern and gain- or loss of function mutation in the different CARD-CC family proteins cause different phenotypes.
- Loss-of-function mutations in CARD9 disrupts lectin receptor signaling like Dectin 1, which causes enhanced susceptibility to fungal infections.[9][10]
- Gain-of-function variants in CARD9 appear to be associated to fibromyalgia, and possibly the comorbidities constipation, Irritable bowel syndrome and other psychosomatic sequelæ in the UK biobank association between exome sequences and health data.[11]
- Strong loss-of-function mutations in CARD11 cause severe defects in lymphocyte function since it is a critical downstream signal mediator in T- and B-cell antigen receptor signaling, which results in severe combined immunodeficiency (SCID).[12]
- Weak (hypomorphic) mutations in CARD11 causes atopic dermatitis disease.[13]
- Gain-of-function mutations in CARD11 can result in B-cell lymphoma or BENTA.[14][15]
- Gain-of-function mutations in CARD14 results in psoriasis or PRP.[16][17]
- There are indications that loss-of-function mutations in CARD14 could result in atopic dermatitis due to disrupted immune responses against skin microbes.[18]
- There are no obvious gain- or loss-of-function mutations in CARD10, but it is sometimes over-expressed in certain cancer variants,[19] and there is a SNP in CARD10 associated to glaucoma.[20]
References
- ↑ "Ancient Origin of the CARD-Coiled Coil/Bcl10/MALT1-Like Paracaspase Signaling Complex Indicates Unknown Critical Functions". Frontiers in Immunology 9: 1136. 2018. doi:10.3389/fimmu.2018.01136. PMID 29881386.
- ↑ Bouchier-Hayes, Lisa; Martin, Seamus J (July 2002). "CARD games in apoptosis and immunity". EMBO Reports 3 (7): 616–621. doi:10.1093/embo-reports/kvf139. ISSN 1469-221X. PMID 12101092. PMC 1084193. https://www.embopress.org/doi/full/10.1093/embo-reports/kvf139. Retrieved 2023-09-11.
- ↑ "CC Protein Domain | Coiled Coil | Cell Signaling Technology". https://www.cellsignal.com/contents/resources-protein-domains-interactions/cc-protein-domain/domains-cc.
- ↑ "The three CARMA sisters: so different, so similar: a portrait of the three CARMA proteins and their involvement in human disorders". Journal of Cellular Physiology 229 (8): 990–7. August 2014. doi:10.1002/jcp.24543. PMID 24375035.
- ↑ Friedrichs, Frauke; Henckaerts, Liesbet; Vermeire, Severine; Kucharzik, Torsten; Seehafer, Tanja; Möller-Krull, Maren; Bornberg-Bauer, Erich; Stoll, Monika et al. (2008-04-01). "The Crohn's disease susceptibility gene DLG5 as a member of the CARD interaction network". Journal of Molecular Medicine 86 (4): 423–432. doi:10.1007/s00109-008-0307-5. ISSN 1432-1440. PMID 18335190. https://doi.org/10.1007/s00109-008-0307-5. Retrieved 2023-09-11.
- ↑ "BioGPS". The Scripps Research Institute. https://biogps.org.
- ↑ Staal, Jens; Driege, Yasmine; Haegman, Mira; Kreike, Marja; Iliaki, Styliani; Vanneste, Domien; Lork, Marie; Afonina, Inna S. et al. (2020-08-13). "Defining the combinatorial space of PKC::CARD-CC signal transduction nodes". The FEBS Journal 288 (5): 1630–1647. doi:10.1111/febs.15522. ISSN 1742-4658. PMID 32790937. https://febs.onlinelibrary.wiley.com/doi/full/10.1111/febs.15522.
- ↑ Gehring, Torben; Seeholzer, Thomas; Krappmann, Daniel (2018). "BCL10 – Bridging CARDs to Immune Activation". Frontiers in Immunology 9: 1539. doi:10.3389/fimmu.2018.01539. ISSN 1664-3224. PMID 30022982.
- ↑ "Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity". Nature 442 (7103): 651–6. August 2006. doi:10.1038/nature04926. PMID 16862125. Bibcode: 2006Natur.442..651G.
- ↑ Online Mendelian Inheritance in Man (OMIM) 607212
- ↑ Jens Staal; Yasmine Driege; Femke Van Gaever; Jill Steels; Rudi Beyaert (2023-01-01). ""Franken-CARD9": chimeric proteins for high-resolution studies of activating mutations in CARD10, CARD11 and CARD14". bioRxiv: 2023–03.06.531260. doi:10.1101/2023.03.06.531260.
- ↑ "Deficiency of caspase recruitment domain family, member 11 (CARD11), causes profound combined immunodeficiency in human subjects". The Journal of Allergy and Clinical Immunology 131 (2): 477–85.e1. February 2013. doi:10.1016/j.jaci.2012.11.050. PMID 23374270. https://openresearch-repository.anu.edu.au/bitstream/1885/11324/7/Stepensky%20et%20al%20%20Deficiency%20of%20caspase%202013.docx.pdf.
- ↑ "Germline hypomorphic CARD11 mutations in severe atopic disease". Nature Genetics 49 (8): 1192–1201. August 2017. doi:10.1038/ng.3898. PMID 28628108.
- ↑ "Germline CARD11 Mutation in a Patient with Severe Congenital B Cell Lymphocytosis". Journal of Clinical Immunology 35 (1): 32–46. January 2015. doi:10.1007/s10875-014-0106-4. PMID 25352053.
- ↑ "Oncogenic CARD11 mutations in human diffuse large B cell lymphoma". Science 319 (5870): 1676–9. March 2008. doi:10.1126/science.1153629. PMID 18323416. Bibcode: 2008Sci...319.1676L.
- ↑ "PSORS2 is due to mutations in CARD14". American Journal of Human Genetics 90 (5): 784–95. May 2012. doi:10.1016/j.ajhg.2012.03.012. PMID 22521418.
- ↑ Online Mendelian Inheritance in Man (OMIM) 607211
- ↑ "Loss-of-function mutations in caspase recruitment domain-containing protein 14 (CARD14) are associated with a severe variant of atopic dermatitis". The Journal of Allergy and Clinical Immunology 143 (1): 173–181.e10. January 2019. doi:10.1016/j.jaci.2018.09.002. PMID 30248356.
- ↑ "CARMA3 Is a Critical Mediator of G Protein-Coupled Receptor and Receptor Tyrosine Kinase-Driven Solid Tumor Pathogenesis". Frontiers in Immunology 9: 1887. 2018. doi:10.3389/fimmu.2018.01887. PMID 30158935.
- ↑ "CARD10 enriched in primary open-angle glaucoma". Molecular Genetics & Genomic Medicine 4 (6): 624–633. November 2016. doi:10.1002/mgg3.248. PMID 27896285.
 |