Biology:Catch bond
A catch bond is a type of noncovalent bond whose dissociation lifetime increases with tensile force applied to the bond. Normally, bond lifetimes are expected to diminish with force.[1] In the case of catch bonds, the lifetime of the bond actually increases up to a maximum before it decreases like in a normal bond. Catch bonds work in a way that is conceptually similar to that of a Chinese finger trap. While catch bonds are strengthened by an increase in force, the force increase is not necessary for the bond to work. Catch bonds were suspected for many years to play a role in the rolling of leukocytes, being strong enough to roll in presence of high forces caused by high shear stresses, while avoiding getting stuck in capillaries where the fluid flow, and therefore shear stress, is low. The existence of catch bonds was debated for many years until strong evidence of their existence was found in bacteria.[2][3] Definite proof of their existence came shortly thereafter in leukocytes.[4]
Discovery
Catch bonds were first proposed in 1988 in the Proceedings of the Royal Society by M. Dembo et al. while at Los Alamos National Laboratory. While developing molecular model to study the critical tension required to detach a membrane bound to a surface through adhesion molecules, it was found that it is theoretically possible for bond dissociation to be increased by force, decreased by force, and independent of force. The terms "slip bond", "catch bond", and "ideal bond" were coined by Dembo to describe these three types of bond behaviors.[5]
Slip bonds represent the ordinary behavior originally modeled by G. Bell, Dembo's former postdoctoral mentor at Los Alamos National Laboratory in 1978.[1] Slip bonds were supported by flow chamber experiments where forces are applied on molecular bonds linking cells to chamber floor under shear flow. By comparison, no decisive evidence of catch bonds was found until 2003. This is due to experimental conditions that were unfavorable for detecting catch bonds, as well as the counterintuitive nature of the bonds themselves. For example, most early experiments were conducted in 96 well plates, an environment that does not provide any flow. Some experiments failed to produce shear stress that is now known to be critical to lengthen the lifetimes of catch bonds, while other experiments conducted under flow conditions too weak or too strong for optimal shear-induced strengthening of these bonds. Finally, Marshall and coworkers found that P-selectin:PSGL-1 bonds exhibited increasing bond lifetime as step loads were applied between 0 and ~10 pN for monomeric interaction but 1 and ~20 pN for dimeric interaction, exhibiting catch bond behavior; after reaching maximum values, which were ~0.6 and 1.2 seconds for monomeric and dimeric interaction, respectively, the bond lifetime fell rapidly at higher loads, displaying slip bond behavior ("catch-slip" bonds).[4] These data were collected using an atomic force microscope and a flow chamber, and have subsequently been duplicated using a biomembrane force probe.[6]
These finding prompted the discoveries of other important catch bonds in the 2000s, including those between L-selectin and PSGL-1 or endoglycan,[7] FimH and mannose,[2] myosin and actin,[8] platelet glycoprotein Ib and von Willebrand factor,[9] and integrin alpha 5 beta 1 and fibronectin.[10] Emphasizing their importance and general acceptance, in the three years following their discovery there were at least 24 articles published on catch bonds.
More catch bonds were discovered in the 2010s, including E-selectin with carbohydrate ligands,[11] G-actin with G-actin or F-actin,[12][13] cadherin-catenin complex with actin,[14] vinculin with F-actin,[15] microtubule with kinetochore particle,[16] integrin alpha L beta 2 and intercellular adhesion molecule 1 (ICAM-1),[17] integrin alpha 4 beta 1 with vascular adhesion molecule 1,[18] integrin alpha M beta 2 with ICAM-1,[19] integrin alpha V beta 3 with fibronectin,[20][21] and integrin alpha IIb beta 3 with fibronectin or fibrinogen.[22]
Sivasankar and his research team have found that the mechanism behind the puzzling phenomenon is due to long-lived, force-induced hydrogen bonds. Using data from previous experiments, the team used molecular dynamics to discover that two rod-shaped cadherins in an X-dimer formed catch bonds when pulled and in the presence of calcium ions.[23] The calcium ions keep the cadherins rigid, while pulling brings the proteins closer together, allowing for hydrogen bonds to form. The mechanism behind catch bonds helps to explain the biophysics behind cell-cell adhesion. According to the researchers, "Robust cadherin adhesion is essential for maintaining the integrity of tissue such as the skin, blood vessels, cartilage and muscle that are exposed to continuous mechanical assault."
The above catch bonds are formed between adhesion receptors and ligands, and among structural molecules and motor proteins, which bear force or generate force in their physiological function. An interesting recent development is the discoveries of catch bonds formed between signaling receptors and their ligands. These include bonds between T cell antigen receptors (TCR) or pre-TCR and peptide presented by major histocompatibility complex (pMHC) molecules,[24][25][26][27][28][29][30] Fc gamma receptor and IgG Fc,[31] and notch receptor and ligands.[32] The presence of catch bonds in the interactions of these signaling (rather than adhesion) receptors have been suggested to be indicative of a possible role of these receptors as mechanoreceptors.[33][34][35]
Variations and related dynamic bonds
Triphasic bonds
Other type of "dynamic bonds" have been defined in additional to the original types of catch bonds, slip bonds and ideal bonds classified by Dembo.[5] Unlike slip bonds, which have been observed in the entire force range tested, catch bonds only exist within certain force range as any molecular bond would eventually be overpowered by high enough force. Therefore, catch bonds are always followed by slip bonds, hence termed "catch-slip bonds". More variations have also been observed, e.g., triphasic slip-catch-slip bonds.[11][36]
Flex bonds
The transition between catch and slip bonds have been modeled as molecular dissociation from two bond states along two pathways.[6] Dissociation along each pathway alone results in a slip bond but at different rates. At low forces, dissociation occurs predominately along the fast pathway. Increasing force tilts the multi-dimensional energy landscape to switch the dissociation from fast pathway to slow pathway, manifesting catch bond. As dissociation along the slow pathway dominates, further increase in force accelerates dissociation, manifesting slip bond. This switching behavior is also called flex bond.[37]
Dynamic catch
The above bonds involve bimolecular interactions, which arguably represents the simplest types. A new type of catch bonds emerges when trimolecular interactions are involved. In such cases, one molecule can interact with the two counter-molecules using two binding sites, either separately, i.e. one at a time in the absence of the other to form bimolecular bonds, or concurrently to form a trimolecular bond when both counter-molecules are present. An interesting finding is that even when the two bimolecular interactions behave as slip bonds, the trimolecular interaction can behave as catch bond. This new type of catch bond, which requires concurrent and cooperative binding, is termed dynamic catch.[38][29]
Cyclic mechanical reinforcement
Most catch bonds were demonstrated using force-clamp force spectroscopy where upon initial ramping, a constant force is loaded on the bond to observe how long the bond lasts, i.e., measuring the bond lifetime at a constant force. Catch bonds are revealed when the mean bond lifetime (reciprocally related to the rate of bond dissociation) increases with the clamped force. Zhu and colleagues demonstrated that bond lifetime measured at the force-clamp phase could be substantially prolonged if the initial ramping included two forms of pre-conditioning: 1) loading the bond by ramping the force to a high level (peak force) before clamping the force at a low level for lifetime measurement, and 2) loading and unloading the bond repeatedly by multiple force cycles before clamping the force at a peak value for lifetime measurement.[39] This new bond type, termed cyclic mechanical reinforcement (CMR), is distinct from catch bond, but it nevertheless resembles catch bond in that the bond lifetime increases with the peak force and with the number of cycles used to pre-condition the bond. CMR has been observed for interactions between integrin alpha 5 beta 1 and fibronectin[39] and between G-actin and G-actin or F-actin.[40]
Force history dependence
The CMR phenomenon indicates that how long a bond can sustain force at a given level can depend on the history of force application prior to arriving at that force level. In other words, the "rate constant" of molecular dissociation at a constant force depends not only on the value of force at the current time but also on the prior force history the bond has experienced in the past. This has indeed been observed for interactions of P-selectin with PSGL-1 or anti-P-selectin antibody,[41] L-selectin with PSGL-1,[42] myosin with actin,[8] integrin alpha V beta 3 with fibrinogen,[43] and TCR with pMHC.[43]
Various catch bonds of specific molecular interactions
Selectin bond
Background
Leukocytes, as well as other types of white blood cells, normally form weak and short-lived bonds with other cells via selectin. Coated outside the membrane of leukocytes are microvilli, which have various types of adhesive molecules, including P-selectin glycoprotein ligand-1 (PSGL-1), a glycoprotein that is normally decorated with sulfated sialyl-Lewis x. the sulfated-sialyl-Lewis-x-contained PSGL-1 molecule has the ability to bind to any type of selectin. Leukocytes also exhibit L-selectin that binds to other cells or other leukocytes that contain PSGL-1 molecules.[44]
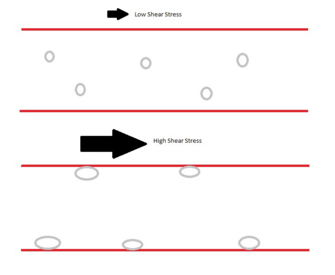
An important example of catch bonds is their role in leukocyte extravasation. During this process, leukocytes move through the circulatory system to sites of infection, and in doing so they 'roll' and bind to selectin molecules on the vessel wall. While able to float freely in the blood under normal circumstances, shear stress induced by inflammation causes leukocytes to attach to the endothelial vessel wall and begin rolling rather than floating downstream. This “shear-threshold phenomenon” was initially characterized in 1996 by Finger et al. who showed that leukocyte binding and rolling through L-selectin is only maintained when a critical shear-threshold is applied to the system.[45] Multiple sources of evidence have shown that catch bonds are responsible for the tether and roll mechanism that allows this critical process to occur. Catch bonds allow increasing force to convert short-lived tethers into stronger, longer-lived binding interactions, thus decreasing the rolling velocity and increasing the regularity of rolling steps. However, this mechanism only works at an optimal force. As shear force increases past this force, bonds revert to slip bonds, creating an increase in velocity and irregularity of rolling.[46]
Leukocytes adhesion mediated by shear stress
In blood vessel, at very low shear stress of ~.3 dynes per squared centimeter, leukocytes do not adhere to the blood vessel endothelial cells. Cells move along the blood vessel at a rate proportional to the blood flow rate. Once the shear stress pass that shear threshold value, leukocytes start to accumulate via selectin binding. At low shear stress above the threshold of about .3 to 5 dynes per squared centimeter, leukocytes alternate between binding and non-binding.[44] Because one leukocyte has many selectins around the surface, these selectin binding/ unbinding cause a rolling motion on the blood vessel. As the shear stress continue to increase, the selectin bonds becomes stronger, causing the rolling velocity to be slower. This reduction in leukocytes rolling velocity allow cells to stop and perform firm binding via integrin binding.[44] Selectin binding do not exhibit "true" catch bond property. Experiments show that at very high shear stress (passing a second threshold), the selectin binding transit between a catch bond to a slip bond binding, in which the rolling velocity increases as the shear force increases.
Leukocyte rolling mediated by catch-slip transition
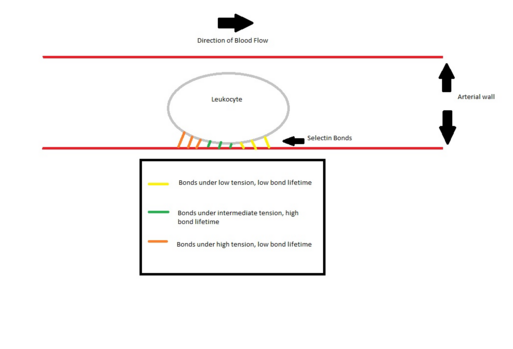
Researchers have hypothesized that the ability of leukocytes to maintain attachment and rolling on the blood vessel wall can be explained by a combination of many factors, including cell flattening to maintain a larger binding surface-area and reduce hydrodynamic drag, as well as tethers holding the rear of the rolling cell to the endothelium breaking and slinging to the front of the rolling cell to reattach to the endothelial wall.[47] These hypotheses work well with Marshall's 2003 findings that selectin bonds go through a catch-slip transition in which initial increases in shear force strengthen the bond, but with enough applied force bond lifetimes begin to decay exponentially.[4] Therefore, the weak binding of a sling at the leading edge of a rolling leukocyte would initially be strengthened as the cell rolls farther and the tension on the bond increases, preventing the cell from dissociating from the endothelial wall and floating freely in the bloodstream despite high shear forces. However, at the trailing edge of the cell, tension becomes high enough to transition the bond from catch to slip, and the bonds tethering the trailing edge eventually break, allowing the cell to roll further instead of remaining stationary.
Proposed mechanisms of action
Allosteric model
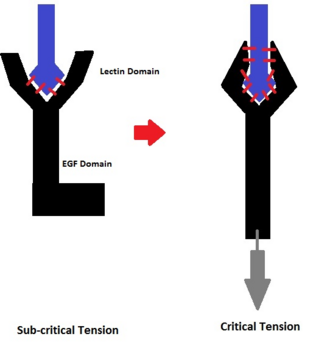
Though catch bonds are now widely recognized, their mechanism of action is still under dispute.[48] Two leading hypotheses dominate the discussion.[citation needed] The first hypothesis, the allosteric model, stems from evidence that x-ray crystallography of selectin proteins shows two conformational states: a bent conformation in the absence of ligand, and an extended conformation in the presence of the ligand.[49] The main domains involved in these states are a lectin domain which contains the ligand binding site and an EGF domain which can shift between bent and extended conformations. The allosteric model claims that tension on the EGF domain favors the extended conformation, and extension of this domain causes a conformational shift in the lectin domain, resulting in greater binding affinity for the ligand.[50] As a result of this conformational change, the ligand is effectively locked in place despite tension exerted on the bond.
Sliding-rebinding model
The sliding-rebinding model differs from the allosteric model in that the allosteric model posits that only one binding site exists and can be altered, but the sliding-rebinding model states that multiple binding sites exist and aren't changed by EGF extension. Rather, in the bent conformation which is favored at low applied forces, the applied force is perpendicular to the line of possible binding sites. Thus, when the association between ligand and lectin domain is interrupted, the bond quickly dissociates. At larger applied forces, however, the protein is extended and the line of possible binding sites is aligned with the applied force, allowing the ligand to quickly re-associate with a new binding site after the initial interaction is disrupted.[51] With multiple binding sites, and even the ability to re-associate with the original binding site, the rate of ligand dissociation would be decreased as is typical of catch bonds.
Mechanism of a single selectin binding
A single PSGL-1 and selectin binding is similar to conventional protein binding when the force is kept constant, with a dissociation constant. As the force exerted starts to increase, the dissociation constant decreases, causing binding to become stronger. As the force reach a threshold level of 11 pN, the dissociation constant starts to increase again, weakening the bond, causing the bond to exhibit a slip bond property.[4]
FimH bond
Background
Catch bonds also play a significant role in bacterial adhesion, most notably in Escherichia coli. E. coli and other bacteria residing in the intestine must be able to adhere to intestinal walls or risk being eliminated from the body through defecation. This is possible due to the bacterial protein FimH, which mediates high adhesion in response to high flow. The lectin domain is one that provides FimH binding the catch bond property when binding to mannose residues from other cells. Experiments have shown that when force is loaded rapidly, bonds were able to survive high forces, thus pointing to catch bond behavior. Catch bonds are responsible for the failure of E. coli in the urinary tract to be eliminated during urination, thus leading to a urinary tract infection. This knowledge is important not only in understanding bacteria, but also for learning how anti-adhesive technologies can be created.[52]
Bacteria adhesion mediated by shear stress
Similar to selectin binding, FimH binding also have a threshold where it only starts binding to the host cells above this threshold. This shear stress threshold is about 1 dynes per squared centimeter, slightly larger than that of selectin binding.[53] Above this threshold, FimH also alternate between binding, pause and unbinding with the mannose residues.[54] However, different from selectin binding, FimH binding to mannose-BSA can either have a very long or very short pauses.[55] This cause FimH binding to exhibit a "stick-and-roll" adhesion, not rolling adhesion in the case of selectin binding.[54] And unlike selectin binding which requires integrin to help with firm adhesion, FimH binding can become stationary, and this process is reversible. All of this is mediated by shear stress level: at shear stress higher than 20 dynes per squared centimeter, FimH binding is stationary. At shear stress higher than 100 dynes per squared centimeter, slow rolling is observed.
See also
- Noncovalent bonding
- Ionic bond
- Hydrogen bond
- Van der Waals force
- Intermolecular force
- Slip bond
References
- ↑ 1.0 1.1 "Models for the specific adhesion of cells to cells". Science 200 (4342): 618–27. May 1978. doi:10.1126/science.347575. PMID 347575. Bibcode: 1978Sci...200..618B.
- ↑ 2.0 2.1 "Bacterial adhesion to target cells enhanced by shear force". Cell 109 (7): 913–23. June 2002. doi:10.1016/S0092-8674(02)00796-1. PMID 12110187.
- ↑ "Dancing with the host: Flow-dependent bacterial adhesion". Cell 110 (1): 1–4. July 2003. doi:10.1016/s0092-8674(02)00821-8. PMID 12150990.
- ↑ 4.0 4.1 4.2 4.3 "Direct observation of catch bonds involving cell-adhesion molecules". Nature 423 (6936): 190–3. May 2003. doi:10.1038/nature01605. PMID 12736689. Bibcode: 2003Natur.423..190M.
- ↑ 5.0 5.1 "The reaction-limited kinetics of membrane-to-surface adhesion and detachment". Proceedings of the Royal Society of London. Series B 234 (1274): 55–83. June 1988. doi:10.1098/rspb.1988.0038. PMID 2901109. Bibcode: 1988RSPSB.234...55D. https://zenodo.org/record/1236126.
- ↑ 6.0 6.1 "Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond". Proceedings of the National Academy of Sciences of the United States of America 101 (31): 11281–6. August 2004. doi:10.1073/pnas.0401870101. PMID 15277675. Bibcode: 2004PNAS..10111281E.
- ↑ "Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan". The Journal of Biological Chemistry 279 (3): 2291–8. January 2004. doi:10.1074/jbc.M310396200. PMID 14573602.
- ↑ 8.0 8.1 "Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction". Proceedings of the National Academy of Sciences of the United States of America 103 (26): 9844–9. June 2006. doi:10.1073/pnas.0601255103. PMID 16785439. Bibcode: 2006PNAS..103.9844G.
- ↑ "Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF". Journal of Clinical Investigation 118 (9): 3195–207. September 2008. doi:10.1172/JCI35754. PMID 18725999.
- ↑ "Demonstration of catch bonds between an integrin and its ligand". Journal of Cell Biology 185 (7): 1275–84. June 2009. doi:10.1083/jcb.200810002. PMID 19564406.
- ↑ 11.0 11.1 "Triphasic force dependence of E-selectin/ligand dissociation governs cell rolling under flow". Biophysical Journal 99 (4): 1166–74. August 2010. doi:10.1016/j.bpj.2010.05.040. PMID 20713000. Bibcode: 2010BpJ....99.1166W.
- ↑ "Actin depolymerization under force is governed by lysine 113:glutamic acid 195-mediated catch-slip bonds". Proceedings of the National Academy of Sciences of the United States of America 110 (13): 5022–27. March 2013. doi:10.1073/Pnas.1218407110. PMID 23460697. Bibcode: 2013PNAS..110.5022L.
- ↑ "Regulation of actin catch-slip bonds with a RhoA-formin module". Scientific Reports 6: 35058. October 2016. doi:10.1038/srep35058. PMID 27731359. Bibcode: 2016NatSR...635058L.
- ↑ "Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force". Science 346 (6209): 1254211. October 2014. doi:10.1126/science.1254211. PMID 25359979.
- ↑ "Vinculin forms a directionally asymmetric catch bond with F-actin". Science 357 (6352): 703–6. August 2017. doi:10.1126/science.aan2556. PMID 28818948. Bibcode: 2017Sci...357..703H.
- ↑ "Tension directly stabilizes reconstituted kinetochore-microtubule attachments". Nature 468 (7323): 576–9. November 2010. doi:10.1038/nature09594. PMID 21107429. Bibcode: 2010Natur.468..576A.
- ↑ "Forcing switch from short- to intermediate- and long-lived states of the alphaA domain generates LFA-1/ICAM-1 catch bonds". Journal of Biological Chemistry 285 (46): 35967–78. September 2010. doi:10.1074/jbc.M110.155770. PMID 20819952.
- ↑ "Dynamic control of beta1 integrin adhesion by the plexinD1-sema3E axis". Proceedings of the National Academy of Sciences of the United States of America 111 (1): 379–84. January 2014. doi:10.1073/pnas.1314209111. PMID 24344262. Bibcode: 2014PNAS..111..379C.
- ↑ "A Lupus-Associated Mac-1 Variant Has Defects in Integrin Allostery and Interaction with Ligands under Force". Cell Reports 10 (10): 1655–64. March 2015. doi:10.1016/j.celrep.2015.02.037. PMID 25772353.
- ↑ "Force regulated conformational change of integrin alphaVbeta3". Matrix Biology 60-61: 70–85. July 2016. doi:10.1016/j.matbio.2016.07.002. PMID 27423389.
- ↑ "Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity". Nature Cell Biology 18 (5): 540–8. May 2016. doi:10.1038/ncb3336. PMID 27065098.
- ↑ "An integrin αIIbβ3 intermediate affinity state mediates biomechanical platelet aggregation". Nature Materials 18 (7): 760–9. July 2019. doi:10.1038/s41563-019-0323-6. PMID 30911119. Bibcode: 2019NatMa..18..760C.
- ↑ Sivasankar, Sanjeevi (2014). "Resolving the molecular mechanism of cadherin catch bond formation". Nature Communications 5: 3941. doi:10.1038/ncomms4941. PMID 24887573. Bibcode: 2014NatCo...5.3941M.
- ↑ "Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling". Cell 157 (2): 357–68. April 2014. doi:10.1016/j.cell.2014.02.053. PMID 24725404.
- ↑ "Force-regulated in situ TCR–peptide-bound MHC class II kinetics determine functions of CD4+ T cells". Journal of Immunology 195 (8): 3557–64. October 2015. doi:10.4049/jimmunol.1501407. PMID 26336148.
- ↑ "Force-dependent transition in the T-cell receptor beta-subunit allosterically regulates peptide discrimination and pMHC bond lifetime". Proceedings of the National Academy of Sciences of the United States of America 112 (5): 1517–22. February 2015. doi:10.1073/pnas.1424829112. PMID 25605925. Bibcode: 2015PNAS..112.1517D.
- ↑ "Pre-T Cell Receptors (Pre-TCRs) Leverage Vbeta Complementarity Determining Regions (CDRs) and Hydrophobic Patch in Mechanosensing Thymic Self-ligands". Journal of Biological Chemistry 291 (49): 25292–305. December 2016. doi:10.1074/jbc.M116.752865. PMID 27707880.
- ↑ Sibener, Leah V.; Fernandes, Ricardo A.; Kolawole, Elizabeth M.; Carbone, Catherine B.; Liu, Fan; McAffee, Darren; Birnbaum, Michael E.; Yang, Xinbo et al. (July 2018). "Isolation of a Structural Mechanism for Uncoupling T Cell Receptor Signaling from Peptide-MHC Binding". Cell 174 (3): 672–687.e27. doi:10.1016/j.cell.2018.06.017. PMID 30053426.
- ↑ 29.0 29.1 Hong, Jinsung; Ge, Chenghao; Jothikumar, Prithiviraj; Yuan, Zhou; Liu, Baoyu; Bai, Ke; Li, Kaitao; Rittase, William et al. (12 November 2018). "A TCR mechanotransduction signaling loop induces negative selection in the thymus". Nature Immunology 19 (12): 1379–1390. doi:10.1038/s41590-018-0259-z. PMID 30420628.
- ↑ "Mechano-regulation of Peptide-MHC Class I Conformations Determines TCR Antigen Recognition". Molecular Cell 73 (5): 1015–27 e7. February 2019. doi:10.1016/j.molcel.2018.12.018. PMID 30711376.
- ↑ Nishi, Hiroshi; Furuhashi, Kazuhiro; Cullere, Xavier; Saggu, Gurpanna; Miller, Mark J.; Chen, Yunfeng; Rosetti, Florencia; Hamilton, Samantha L. et al. (11 September 2017). "Neutrophil FcγRIIA promotes IgG-mediated glomerular neutrophil capture via Abl/Src kinases". Journal of Clinical Investigation 127 (10): 3810–3826. doi:10.1172/JCI94039. PMID 28891817.
- ↑ Luca, Vincent C.; Kim, Byoung Choul; Ge, Chenghao; Kakuda, Shinako; Wu, Di; Roein-Peikar, Mehdi; Haltiwanger, Robert S.; Zhu, Cheng et al. (24 March 2017). "Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity". Science 355 (6331): 1320–1324. doi:10.1126/science.aaf9739. PMID 28254785. Bibcode: 2017Sci...355.1320L.
- ↑ "The structural basis of αβ T-lineage immune recognition: TCR docking topologies, mechanotransduction, and co-receptor function". Immunological Reviews 250 (1): 102–19. November 2012. doi:10.1111/j.1600-065X.2012.01161.x. PMID 23046125.
- ↑ Chen, Yunfeng; Ju, Lining; Rushdi, Muaz; Ge, Chenghao; Zhu, Cheng; Weaver, Valerie Marie (7 November 2017). "Receptor-mediated cell mechanosensing". Molecular Biology of the Cell 28 (23): 3134–3155. doi:10.1091/mbc.E17-04-0228. PMID 28954860.
- ↑ Zhu, Cheng; Chen, Wei; Lou, Jizhong; Rittase, William; Li, Kaitao (18 September 2019). "Mechanosensing through immunoreceptors". Nature Immunology 20 (10): 1269–1278. doi:10.1038/s41590-019-0491-1. PMID 31534240.
- ↑ "The N-terminal flanking region of the A1 domain regulates the force-dependent binding of von Willebrand factor to platelet glycoprotein Ibalpha". Journal of Biological Chemistry 288 (45): 32289–301. November 2013. doi:10.1074/jbc.M113.504001. PMID 24062306.
- ↑ "A mechanically stabilized receptor-ligand flex-bond important in the vasculature". Nature 466 (7309): 992–5. August 2010. doi:10.1038/nature09295. PMID 20725043. Bibcode: 2010Natur.466..992K.
- ↑ "Dynamic catch of a Thy-1–α5β1+ syndecan-4 trimolecular complex". Nature Communications 466 (7309): 4886. September 2014. doi:10.1038/ncomms5886. PMID 25216363. Bibcode: 2014NatCo...5.4886F.
- ↑ 39.0 39.1 "Cyclic mechanical reinforcement of integrin-ligand interactions". Molecular Cell 49 (6): 1060–8. February 2013. doi:10.1016/j.molcel.2013.01.015. PMID 23416109.
- ↑ "Force-history dependence and cyclic mechanical reinforcement of actin filaments at the single molecular level". Journal of Cell Science 132 (4): jcs216911. February 2013. doi:10.1242/jcs.216911. PMID 30659118.
- ↑ "Force history dependence of receptor-ligand dissociation". Biophysical Journal 88 (2): 1458–66. November 2005. doi:10.1529/biophysj.104.050567. PMID 15556978. Bibcode: 2005BpJ....88.1458M.
- ↑ "Regulation of catch bonds by rate of force application". Journal of Biological Chemistry 286 (37): 32749–61. July 2011. doi:10.1074/jbc.M111.240044. PMID 21775439.
- ↑ 43.0 43.1 "Fast force loading disrupts molecular binding stability in human and mouse cell adhesions". Molecular & Cellular Biomechanics 16 (3): 211–23. December 2019. doi:10.32604/mcb.2019.07267.
- ↑ 44.0 44.1 44.2 Thomas, Wendy (August 2008). "Catch Bonds in Adhesion". Annual Review of Biomedical Engineering 10 (1): 39–57. doi:10.1146/annurev.bioeng.10.061807.160427. PMID 18647111.
- ↑ Finger, Erik B.; Purl, Kamal D.; Alon, Ronen; Lawrence, Michael B.; von Andrian, Ulrich H.; Springer, Timothy A. (January 1996). "Adhesion through L-selectin requires a threshold hydrodynamic shear". Nature 379 (6562): 266–269. doi:10.1038/379266a0. PMID 8538793. Bibcode: 1996Natur.379..266F.
- ↑ Zhu, Cheng; Lou, Jizhong; McEver, Rodger P. (1 January 2005). "Catch bonds: Physical models, structural bases, biological function and rheological relevance". Biorheology 42 (6): 443–462. PMID 16369083. https://content.iospress.com/articles/biorheology/bir378.
- ↑ Sundd, Prithu; Pospieszalska, Maria K.; Ley, Klaus (August 2013). "Neutrophil rolling at high shear: Flattening, catch bond behavior, tethers and slings". Molecular Immunology 55 (1): 59–69. doi:10.1016/j.molimm.2012.10.025. PMID 23141302.
- ↑ "Activated nanoscale actin-binding domain motion in the catenin-cadherin complex revealed by neutron spin echo spectroscopy". Proc Natl Acad Sci USA 118 (13): e2025012118. March 30, 2021. doi:10.1073/pnas.2025012118. PMID 33753508. Bibcode: 2021PNAS..11825012F.
- ↑ Somers, William S; Tang, Jin; Shaw, Gray D; Camphausen, Raymond T (October 2000). "Insights into the Molecular Basis of Leukocyte Tethering and Rolling Revealed by Structures of P- and E-Selectin Bound to SLeX and PSGL-1". Cell 103 (3): 467–479. doi:10.1016/s0092-8674(00)00138-0. PMID 11081633.
- ↑ Waldron, T. T.; Springer, T. A. (31 December 2008). "Transmission of allostery through the lectin domain in selectin-mediated cell adhesion". Proceedings of the National Academy of Sciences 106 (1): 85–90. doi:10.1073/pnas.0810620105. PMID 19118202. Bibcode: 2009PNAS..106...85W.
- ↑ Lou, Jizhong; Yago, Tadayuki; Klopocki, Arkadiusz G.; Mehta, Padmaja; Chen, Wei; Zarnitsyna, Veronika I.; Bovin, Nicolai V.; Zhu, Cheng et al. (25 September 2006). "Flow-enhanced adhesion regulated by a selectin interdomain hinge". Journal of Cell Biology 174 (7): 1107–1117. doi:10.1083/jcb.200606056. PMID 17000883.
- ↑ Yakovenko, Olga; Sharma, Shivani; Forero, Manu; Tchesnokova, Veronika; Aprikian, Pavel; Kidd, Brian; Mach, Albert; Vogel, Viola et al. (2008). "FimH Forms Catch Bonds That Are Enhanced by Mechanical Force Due to Allosteric Regulation". The Journal of Biological Chemistry 283 (17): 11596–11605. doi:10.1074/jbc.m707815200. PMID 18292092.
- ↑ Thomas, Wendy E. (2005). "Using a Laminar Flow System to Explain Shear-Enhanced Bacterial Adhesion". ASME 3rd International Conference on Microchannels and Minichannels, Parts a and B. pp. 751–759. doi:10.1115/ICMM2005-75217. ISBN 0-7918-4185-5.
- ↑ 54.0 54.1 Thomas, Wendy E.; Nilsson, Lina M.; Forero, Manu; Sokurenko, Evgeni V.; Vogel, Viola (3 August 2004). "Shear-dependent 'stick-and-roll' adhesion of type 1 fimbriated Escherichia coli". Molecular Microbiology 53 (5): 1545–1557. doi:10.1111/j.1365-2958.2004.04226.x. PMID 15387828.
- ↑ Thomas, Wendy; Forero, Manu; Yakovenko, Olga; Nilsson, Lina; Vicini, Paolo; Sokurenko, Evgeni; Vogel, Viola (February 2006). "Catch-Bond Model Derived from Allostery Explains Force-Activated Bacterial Adhesion". Biophysical Journal 90 (3): 753–764. doi:10.1529/biophysj.105.066548. PMID 16272438. Bibcode: 2006BpJ....90..753T.
 |
