Biology:Cyclodeaminase domain
| FTCD_C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
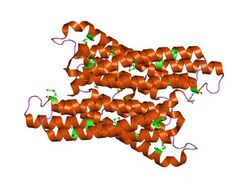 crystal structure of formiminotetrahydrofolate cyclodeaminase (tm1560) from thermotoga maritima at 2.80 a resolution | |||||||||
| Identifiers | |||||||||
| Symbol | FTCD_C | ||||||||
| Pfam | PF04961 | ||||||||
| InterPro | IPR007044 | ||||||||
| SCOP2 | 1o5h / SCOPe / SUPFAM | ||||||||
| |||||||||
In molecular biology, enzymes containing the cyclodeaminase domain function in channeling one-carbon units to the folate pool. In most cases, this domain acts as a formimidoyltetrahydrofolate cyclodeaminase, which catalyses the cyclisation of formimidoyltetrahydrofolate to methenyltetrahydrofolate as shown in reaction (1). In the methylotrophic bacterium Methylobacterium extorquens, however, it acts as a methenyltetrahydrofolate cyclohydrolase, which catalyses the interconversion of formyltetrahydrofolate and methylenetetrahydrofolate, as shown in reaction (2).[1]
(1) 5-formimidoyltetrahydrofolate = 5,10-methenyltetrahydrofolate + NH(3)
(2) 10- formyltetrahydrofolate = 5,10-methenyltetrahydrofolate + H(2)O
In prokaryotes, this domain mostly occurs on its own, while in eukaryotes it is fused to a glutamate formiminotransferase domain (which catalyses the previous step in the pathway) to form the bifunctional enzyme formiminotransferase cyclodeaminase.[2] The eukaryotic enzyme is a circular tetramer of homodimers, while the prokaryotic enzyme is a dimer.[1][3][4]
The crystal structure of the cyclodeaminase enzyme from Thermaotogoa maritima has been studied.[4] It is a homodimer, where each monomer is composed of six alpha helices arranged in an up and down helical bundle, forming a novel fold. The location of the active site is not known, but sequence alignments revealed two clusters of conserved residues located in a deep pocket within the dimmer interface. This pocket was large enough to accommodate the reaction product and it was postulated that this is the active site.
References
- ↑ 1.0 1.1 "A methenyl tetrahydromethanopterin cyclohydrolase and a methenyl tetrahydrofolate cyclohydrolase in Methylobacterium extorquens AM1". Eur. J. Biochem. 261 (2): 475–80. April 1999. doi:10.1046/j.1432-1327.1999.00291.x. PMID 10215859.
- ↑ "The two monofunctional domains of octameric formiminotransferase-cyclodeaminase exist as dimers". Biochemistry 34 (33): 10358–64. August 1995. doi:10.1021/bi00033a006. PMID 7654689.
- ↑ "The bifunctional enzyme formiminotransferase-cyclodeaminase is a tetramer of dimers". J. Biol. Chem. 255 (19): 9474–8. October 1980. doi:10.1016/S0021-9258(19)70586-9. PMID 7410436.
- ↑ 4.0 4.1 "Crystal structure of a formiminotetrahydrofolate cyclodeaminase (TM1560) from Thermotoga maritima at 2.80 A resolution reveals a new fold". Proteins 58 (4): 976–81. March 2005. doi:10.1002/prot.20364. PMID 15651027.
 |

