Biology:Cytochrome b5
| Cytochrome b5 | |
|---|---|
 | |
| Identifiers | |
| Symbol | CYB5A |
| Alt. symbols | CYB5 |
| NCBI gene | 1528 |
| HGNC | 2570 |
| OMIM | 250790 |
| PDB | 1JEX |
| RefSeq | NM_001914 |
| UniProt | P00167 |
| Other data | |
| Locus | Chr. 18 q23 |
| Cytochrome b5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Cyt_B5 | ||||||||
| Pfam | PF00173 | ||||||||
| InterPro | IPR001199 | ||||||||
| PROSITE | PDOC00170 | ||||||||
| Membranome | 211 | ||||||||
| |||||||||
Cytochromes b5 are ubiquitous electron transport hemoproteins found in animals, plants, fungi and purple phototrophic bacteria. The microsomal and mitochondrial variants are membrane-bound, while bacterial and those from erythrocytes and other animal tissues are water-soluble. The family of cytochrome b5-like proteins includes (besides cytochrome b5 itself) hemoprotein domains covalently associated with other redox domains in flavocytochrome cytochrome b2 (L-lactate dehydrogenase; EC 1.1.2.3), sulfite oxidase (EC 1.8.3.1), plant and fungal nitrate reductases (EC 1.7.1.1, EC 1.7.1.2, EC 1.7.1.3), and plant and fungal cytochrome b5/acyl lipid desaturase fusion proteins.
Structure
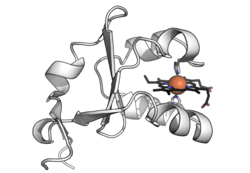
3-D structures of a number of cytochrome b5 and yeast flavocytochrome b2 are known. The fold belongs to the α+β class, with two hydrophobic cores on each side of a β-sheet. The larger hydrophobic core constitutes the heme-binding pocket, closed off on each side by a pair of helices connected by a turn. The smaller hydrophobic core may have only a structural role and is formed by spatially close N-terminal and C-terminal segments. The two histidine residues provide the fifth and sixth heme ligands, and the propionate edge of the heme group lies at the opening of the heme crevice. Two isomers of cytochrome b5, referred to as the A (major) and B (minor) forms, differ by a 180° rotation of the heme about an axis defined by the α- and γ-meso carbons.
Cytochrome b5 in some biochemical reactions
EC 1.6.2.2 cytochrome-b5 reductase
- NADH + H+ + 2 ferricytochrome b5 → NAD+ + 2 ferrocytochrome b5
EC 1.10.2.1 L-ascorbate—cytochrome-b5 reductase
- L-ascorbate + ferricytochrome b5 → monodehydroascorbate + ferrocytochrome b5
EC 1.14.18.2 CMP-N-acetylneuraminate monooxygenase
- CMP-N-acetylneuraminate + 2 ferrocytochrome b5 + O2 + 2 H+ → CMP-N-glycoloylneuraminate + 2 ferricytochrome b5 + H2O
EC 1.14.19.1 stearoyl-CoA 9-desaturase
- stearoyl-CoA + 2 ferrocytochrome b5 + O2 + 2 H+ → oleoyl-CoA + 2 ferricytochrome b5 + H2O
EC 1.14.19.3 linoleoyl-CoA 9-desaturase
- linoleoyl-CoA + 2 ferrocytochrome b5 + O2 + 2 H+ → γ-linolenoyl-CoA + 2 ferricytochrome b5 + H2O
See also
References
- "The cytochrome b5-fold: an adaptable module". Biochimie 76 (7): 674–92. 1994. doi:10.1016/0300-9084(94)90144-9. PMID 7893819.
- "The role of cytochrome b5 fusion desaturases in the synthesis of polyunsaturated fatty acids". Prostaglandins, Leukotrienes, and Essential Fatty Acids 68 (2): 135–43. February 2003. doi:10.1016/S0952-3278(02)00263-6. PMID 12538077.
- "Gene synthesis, bacterial expression, and 1H NMR spectroscopic studies of the rat outer mitochondrial membrane cytochrome b5". Biochemistry 31 (48): 12233–40. December 1992. doi:10.1021/bi00163a037. PMID 1333795.
- "The many roles of cytochrome b5". Pharmacology & Therapeutics 97 (2): 139–52. February 2003. doi:10.1016/S0163-7258(02)00327-3. PMID 12559387.
External links
- PDB: 1B5A – Solution structure of rat cytochrome b5 (form A)
- PDB: 1B5B – Solution structure of rat cytochrome b5 (form B)
- PDB: 1CXY – X-ray structure of cytochrome b558 from Ectothiorhodospira vacuolata
- Online Mendelian Inheritance in Man (OMIM) 250790 – Methemoglobinemia due to deficiency of cytochrome b5
 |
