Biology:Endosphere
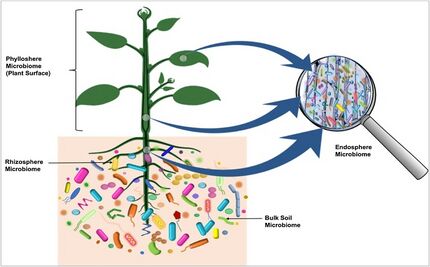
Some microorganisms, such as endophytes, penetrate and occupy the plant internal tissues, forming the endospheric microbiome. The arbuscular mycorrhizal and other endophytic fungi are the dominant colonizers of the endosphere.[2] Bacteria, and to some degree archaea, are important members of endosphere communities. Some of these endophytic microbes interact with their host and provide obvious benefits to plants.[3][4][5] Unlike the rhizosphere and the rhizoplane, the endospheres harbor highly specific microbial communities. The root endophytic community can be very distinct from that of the adjacent soil community. In general, diversity of the endophytic community is lower than the diversity of the microbial community outside the plant.[6] The identity and diversity of the endophytic microbiome of above-and below-ground tissues may also differ within the plant.[7][2][1]
Leaves and bacteria
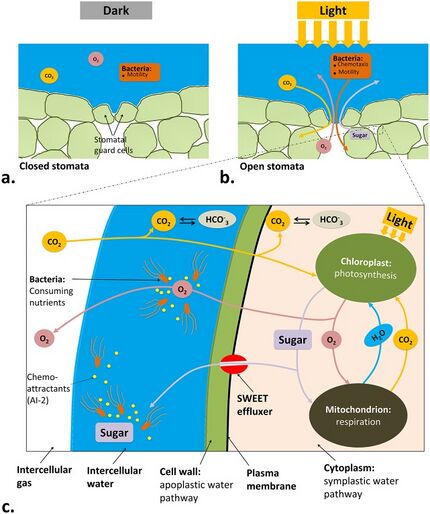
Physical schematic focusing on a stomatal opening under
a) dark conditions b) light conditions
c) Closeup of underlying pathways within a leaf tissue
that result in light-induced bacterial infiltration into the leaf
Exposure to light can trigger photosynthesis in plant leaves, such as leafy-greens, and increase concentrations of photosynthetic products, such as glucose, within the leaf tissue. Bacteria existing at the leaf surfaces may respond to the available photosynthetic products and migrate into the leaf tissue by chemotaxis toward nutrient concentration gradients. Once the bacteria are inside the leaf tissue, they cannot be washed away, presenting a risk to consumers.[8] Several bacteria, such as Escherichia coli and Salmonella enterica, are able to attach the microstructure at the surface of plant leaves, such as trichomes, stomata and grooves,[9] and localize at sites that are not accessible for wash water and sanitizers. The bacteria are also able to infiltrate into available openings at the leaf surface, such as stomata, cuts and wounds, to reach tens of micrometer depths below the leaf epidermis.[10] This infiltration can present a risk to human consumption of raw leafy greens.[11][8]
Light is one of the driving forces that can promote infiltration of pathogenic bacteria into plant leaves. Incubation of S. enterica (serovar Typhimurium) on iceberg lettuce leaves in the light led to association of bacteria near open stomata and infiltration into the leaf tissue. However, a dark condition caused a scattered attachment pattern at the leaf surface and a poor stomatal infiltration.[10] Nutrients, such as glucose and sucrose, produced by photosynthetically active cells in the leaf tissue during light exposure are attractive for bacteria that may be initially present at the leaf surface.[12] Opening of the stomata in light brings up an opportunity for bacteria to transport via chemotaxis toward the gradients of nutrients into the leaf interior. Many plants have evolved stomatal defense machinery to close the stomata upon perception of bacterial surface structures, known as microbe-associated molecular patterns (MAMPs).[13] However, it is not always successful and some human pathogens were shown to penetrate the leaf interior through a process involved with chemotaxis and motility.[10][8]
References
- ↑ 1.0 1.1 Dastogeer, Khondoker M.G.; Tumpa, Farzana Haque; Sultana, Afruja; Akter, Mst Arjina; Chakraborty, Anindita (2020). "Plant microbiome–an account of the factors that shape community composition and diversity". Current Plant Biology 23. doi:10.1016/j.cpb.2020.100161. Bibcode: 2020CPBio..2300161D. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ 2.0 2.1 Vokou, Despoina; Vareli, Katerina; Zarali, Ekaterini; Karamanoli, Katerina; Constantinidou, Helen-Isis A.; Monokrousos, Nikolaos; Halley, John M.; Sainis, Ioannis (2012). "Exploring Biodiversity in the Bacterial Community of the Mediterranean Phyllosphere and its Relationship with Airborne Bacteria". Microbial Ecology 64 (3): 714–724. doi:10.1007/s00248-012-0053-7. PMID 22544345. Bibcode: 2012MicEc..64..714V.
- ↑ Bulgarelli, Davide; Rott, Matthias; Schlaeppi, Klaus; Ver Loren Van Themaat, Emiel; Ahmadinejad, Nahal; Assenza, Federica; Rauf, Philipp; Huettel, Bruno et al. (2012). "Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota". Nature 488 (7409): 91–95. doi:10.1038/nature11336. PMID 22859207. Bibcode: 2012Natur.488...91B.
- ↑ Dastogeer, Khondoker M.G.; Li, Hua; Sivasithamparam, Krishnapillai; Jones, Michael G.K.; Du, Xin; Ren, Yonglin; Wylie, Stephen J. (2017). "Metabolic responses of endophytic Nicotiana benthamiana plants experiencing water stress". Environmental and Experimental Botany 143: 59–71. doi:10.1016/j.envexpbot.2017.08.008. Bibcode: 2017EnvEB.143...59D. https://researchrepository.murdoch.edu.au/id/eprint/38407/.
- ↑ Rodriguez, R. J.; White Jr, J. F.; Arnold, A. E.; Redman, R. S. (2009). "Fungal endophytes: Diversity and functional roles". New Phytologist 182 (2): 314–330. doi:10.1111/j.1469-8137.2009.02773.x. PMID 19236579. Bibcode: 2009NewPh.182..314R.
- ↑ Schlaeppi, K.; Dombrowski, N.; Oter, R. G.; Ver Loren Van Themaat, E.; Schulze-Lefert, P. (2014). "Quantitative divergence of the bacterial root microbiota in Arabidopsis thaliana relatives". Proceedings of the National Academy of Sciences 111 (2): 585–592. doi:10.1073/pnas.1321597111. PMID 24379374. Bibcode: 2014PNAS..111..585S.
- ↑ Abdelfattah, Ahmed; Wisniewski, Michael; Schena, Leonardo; Tack, Ayco J.M. (2020-05-14) (in en). Experimental Evidence of Microbial Inheritance in Plants and Transmission Routes from Seed to Phyllosphere and Root. doi:10.21203/rs.3.rs-27656/v1. https://www.researchsquare.com/article/rs-27656/v1.
- ↑ 8.0 8.1 8.2 8.3 Ranjbaran, Mohsen; Solhtalab, Mina; Datta, Ashim K. (2020). "Mechanistic modeling of light-induced chemotactic infiltration of bacteria into leaf stomata". PLOS Computational Biology 16 (5). doi:10.1371/journal.pcbi.1007841. PMID 32384085. Bibcode: 2020PLSCB..16E7841R. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Warning, Alexander D.; Datta, Ashim K. (2017). "Mechanistic understanding of non-spherical bacterial attachment and deposition on plant surface structures". Chemical Engineering Science 160: 396–418. doi:10.1016/j.ces.2016.11.030. Bibcode: 2017ChEnS.160..396W.
- ↑ 10.0 10.1 10.2 Kroupitski, Yulia; Golberg, Dana; Belausov, Eduard; Pinto, Riky; Swartzberg, Dvora; Granot, David; Sela, Shlomo (2009). "Internalization of Salmonella enterica in Leaves is Induced by Light and Involves Chemotaxis and Penetration through Open Stomata". Applied and Environmental Microbiology 75 (19): 6076–6086. doi:10.1128/AEM.01084-09. PMID 19648358. Bibcode: 2009ApEnM..75.6076K.
- ↑ Olaimat, Amin N.; Holley, Richard A. (2012). "Factors influencing the microbial safety of fresh produce: A review". Food Microbiology 32 (1): 1–19. doi:10.1016/j.fm.2012.04.016. PMID 22850369.
- ↑ Golberg, Dana; Kroupitski, Yulia; Belausov, Eduard; Pinto, Riky; Sela, Shlomo (2011). "Salmonella Typhimurium internalization is variable in leafy vegetables and fresh herbs". International Journal of Food Microbiology 145 (1): 250–257. doi:10.1016/j.ijfoodmicro.2010.12.031. PMID 21262550.
- ↑ Melotto, Maeli; Panchal, Shweta; Roy, Debanjana (2014). "Plant innate immunity against human bacterial pathogens". Frontiers in Microbiology 5: 411. doi:10.3389/fmicb.2014.00411. PMID 25157245.
 |
