Biology:L-type lectin domain
| Lectin_leg-like | |||||||||
|---|---|---|---|---|---|---|---|---|---|
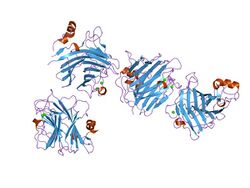 the crystal structure of the carbohydrate recognition domain of the glycoprotein sorting receptor p58/ergic-53 reveals a novel metal binding site and conformational changes associated with calcium ion binding | |||||||||
| Identifiers | |||||||||
| Symbol | Lectin_leg-like | ||||||||
| Pfam | PF03388 | ||||||||
| Pfam clan | CL0004 | ||||||||
| InterPro | IPR005052 | ||||||||
| SCOP2 | 1gv9 / SCOPe / SUPFAM | ||||||||
| Membranome | 719 | ||||||||
| |||||||||
In molecular biology the L-like lectin domain is a protein domain found in lectins which are similar to the leguminous plant lectins.
Lectins are structurally diverse proteins that bind to specific carbohydrates. This family includes the VIP36 and ERGIC-53 lectins.[1] Although proteins containing this domain were originally identified as a family of animal lectins, there are also yeast representatives.[1]
ERGIC-53 is a 53kDa protein, localised to the intermediate region between the endoplasmic reticulum and the Golgi apparatus (ER-Golgi-Intermediate Compartment, ERGIC). It was identified as a calcium-dependent, mannose-specific lectin.[2] Its dysfunction has been associated with combined factors V and VIII deficiency, suggesting an important and substrate-specific role for ERGIC-53 in the glycoprotein-secreting pathway.[2][3]
The L-like lectin domain has an overall globular shape composed of a beta-sandwich of two major twisted antiparallel beta-sheets. The beta-sandwich comprises a major concave beta-sheet and a minor convex beta-sheet, in a variation of the jelly roll fold.[4][5][6][7]
References
- ↑ Jump up to: 1.0 1.1 "A putative novel class of animal lectins in the secretory pathway homologous to leguminous lectins". Cell 77 (5): 625–6. June 1994. doi:10.1016/0092-8674(94)90047-7. PMID 8205612.
- ↑ Jump up to: 2.0 2.1 "ERGIC-53 is a functional mannose-selective and calcium-dependent human homologue of leguminous lectins". Mol. Biol. Cell 7 (3): 483–93. March 1996. doi:10.1091/mbc.7.3.483. PMID 8868475.
- ↑ "ERGIC-53 gene structure and mutation analysis in 19 combined factors V and VIII deficiency families". Blood 93 (7): 2261–6. April 1999. PMID 10090935.
- ↑ "Crystal structure of the carbohydrate recognition domain of p58/ERGIC-53, a protein involved in glycoprotein export from the endoplasmic reticulum". J. Biol. Chem. 277 (18): 15979–84. May 2002. doi:10.1074/jbc.M112098200. PMID 11850423.
- ↑ "The crystal structure of the carbohydrate-recognition domain of the glycoprotein sorting receptor p58/ERGIC-53 reveals an unpredicted metal-binding site and conformational changes associated with calcium ion binding". J. Mol. Biol. 334 (5): 845–51. December 2003. doi:10.1016/j.jmb.2003.10.031. PMID 14643651.
- ↑ "Structures of the carbohydrate recognition domain of Ca2+-independent cargo receptors Emp46p and Emp47p". J. Biol. Chem. 281 (15): 10410–9. April 2006. doi:10.1074/jbc.M512258200. PMID 16439369.
- ↑ "Structural basis for recognition of high mannose type glycoproteins by mammalian transport lectin VIP36". J. Biol. Chem. 282 (38): 28246–55. September 2007. doi:10.1074/jbc.M703064200. PMID 17652092. http://espace.library.uq.edu.au/view/UQ:130028/UQ130028_OA.pdf.
 |

