Biology:LINE1
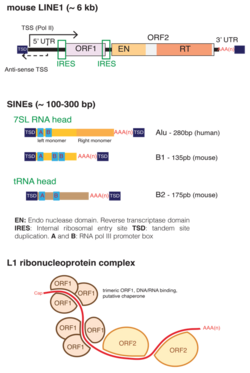
LINE1 (also L1 and LINE-1) is a family of related class I transposable elements in the DNA of some organisms, classified with the long interspersed elements (LINEs). L1 transposons comprise approximately 17% of the human genome.[1] These active L1s can interrupt the genome through insertions, deletions, rearrangements, and copy number variations.[2] L1 activity has contributed to the instability and evolution of genomes and is tightly regulated in the germline by DNA methylation, histone modifications, and piRNA.[3] L1s can further impact genome variation through mispairing and unequal crossing over during meiosis due to its repetitive DNA sequences.[2]
L1 gene products are also required by many non-autonomous Alu and SVA SINE retrotransposons. Mutations induced by L1 and its non-autonomous counterparts have been found to cause a variety of heritable and somatic diseases.[4][5]
In 2011, human L1 was reportedly discovered in the genome of the gonorrhea bacteria, evidently having arrived there by horizontal gene transfer.[6][7]
Structure
A typical L1 element is approximately 6,000 base pairs (bp) long and consists of two non-overlapping open reading frames (ORFs) which are flanked by untranslated regions (UTRs) and target site duplications. In humans, ORF2 is thought to be translated by an unconventional termination/reinitiation mechanism,[8] while mouse L1s contain an internal ribosome entry site (IRES) upstream of each ORF.[9]
5' UTR
The 5' UTRs of mouse L1s contain a variable number of GC-rich tandemly repeated monomers of around 200 bp, followed by a short non-monomeric region. Human 5’ UTRs are ~900 bp in length and do not contain repeated motifs. All families of human L1s harbor in their most 5’ extremity a binding motif for the transcription factor YY1.[10] Younger families also have two binding sites for SOX-family transcription factors, and both YY1 and SOX sites were shown to be required for human L1 transcription initiation and activation.[11][12] Both mouse and human 5’ UTRs also contain a weak antisense promoter of unknown function.[13][14]
ORF1
| LINE-1 (L1.2) retrotransposable element ORF1 | |
|---|---|
| Identifiers | |
| Symbol | L1RE1 |
| Alt. symbols | L1ORF1p |
| NCBI gene | 4029 |
| HGNC | 6686 |
| OMIM | 151626 |
| PDB | 2LDY |
| UniProt | Q9UN81 |
| Other data | |
| Locus | Chr. 22 q12.1 |
| Wikidata | Q18028646 |
The first ORF of L1 encodes a 500-amino acid, 40-kDa protein that lacks homology with any protein of known function. In vertebrates, it contains a conserved C-terminus domain and a highly variable coiled-coil N-terminus that mediates the formation of ORF1 trimeric complexes. ORF1 trimers have RNA-binding and nucleic acid chaperone activity that are necessary for retrotransposition.[15]
ORF2
| LINE-1 retrotransposable element ORF2 | |
|---|---|
| Identifiers | |
| Symbol | L1RE2 |
| Alt. symbols | L1ORF2p |
| NCBI gene | 4030 |
| HGNC | 6687 |
| PDB | 1VYB |
| UniProt | O00370 |
| Other data | |
| Locus | Chr. 1 q |
| Wikidata | Q18028649 |
The second ORF of L1 encodes a protein that has endonuclease and reverse transcriptase activity. The encoded protein has a molecular weight of 150 kDa. The structure of the ORF2 protein was solved in 2023. Its protein core contains three domains of unknown functions, termed "tower/EN-linker" and "wrist/RNA-binding domain" that bind Alu RNA's polyA tail and C-terminal domain that binds Alu RNA stem loop.
The nicking and reverse transcriptase activities of L1 ORF2p are boosted by single-stranded DNA structures likely present on the active replication forks. Unlike viral RTs, L1 ORF2p can be primed by RNA, including RNA hairpin primers produced by the Alu element.
Regulation
As with other transposable elements, the host organism keeps a heavy check on LINE1 to prevent it from becoming overly active. In the primitive eukaryote Entamoeba histolytica, ORF2 is massively expressed in antisense, resulting in no detectable amounts of its protein product.[16]
Roles in disease
Cancer
L1 activity has been observed in numerous types of cancers, with particularly extensive insertions found in colorectal and lung cancers.[17] It is currently unclear if these insertions are causes or secondary effects of cancer progression. However, at least two cases have found somatic L1 insertions causative of cancer by disrupting the coding sequences of genes APC and PTEN in colon and endometrial cancer, respectively.[2]
Quantification of L1 copy number by qPCR or L1 methylation levels with bisulfite sequencing are used as diagnostic biomarkers in some types of cancers. L1 hypomethylation of colon tumor samples is correlated with cancer stage progression.[18][19] Furthermore, less invasive blood assays for L1 copy number or methylation levels are indicative of breast or bladder cancer progression and may serve as methods for early detection.[20][21]
Neuropsychiatric disorders
Higher L1 copy numbers have been observed in the human brain compared to other organs.[22][23] Studies of animal models and human cell lines have shown that L1s become active in neural progenitor cells (NPCs), and that experimental deregulation of or overexpression of L1 increases somatic mosaicism. This phenomenon is negatively regulated by Sox2, which is downregulated in NPCs, and by MeCP2 and methylation of the L1 5' UTR.[24] Human cell lines modeling the neurological disorder Rett syndrome, which carry MeCP2 mutations, exhibit increased L1 transposition, suggesting a link between L1 activity and neurological disorders.[25][24] Current studies are aimed at investigating the potential roles of L1 activity in various neuropsychiatric disorders including schizophrenia, autism spectrum disorders, epilepsy, bipolar disorder, Tourette syndrome, and drug addiction.[26] L1s are also highly expressed in octopus brain, suggesting a convergent mechanism in complex cognition.[27]
Retinal disease
Increased RNA levels of Alu, which requires L1 proteins, are associated with a form of age-related macular degeneration, a neurological disorder of the eyes.[28]
The naturally occurring mouse retinal degeneration model rd7 is caused by an L1 insertion in the Nr2e3 gene.[29]
Assistance to telomere reprogramming
It has been suggested that L1s may directly contribute to telomere reprogramming at the 2-cell stage of embryo development.[30][31]
COVID-19
In 2021, a study proposed that L1 elements may be responsible for potential endogenisation of the SARS-CoV-2 genome in Huh7 mutant cancer cells,[32] which would possibly explain why some patients test PCR positive for SARS-CoV-2 even after clearance of the virus. These results however have been criticized as not reproducible,[33] misleading and infrequent[34] or artefactual.[35]
See also
References
- ↑ "Initial sequencing and analysis of the human genome". Nature 409 (6822): 860–921. February 2001. doi:10.1038/35057062. PMID 11237011. Bibcode: 2001Natur.409..860L.
- ↑ 2.0 2.1 2.2 "Mobile DNA in Health and Disease". The New England Journal of Medicine 377 (4): 361–370. July 2017. doi:10.1056/NEJMra1510092. PMID 28745987.
- ↑ "Tracking LINE1 retrotransposition in the germline". Proceedings of the National Academy of Sciences of the United States of America 114 (28): 7194–7196. July 2017. doi:10.1073/pnas.1709067114. PMID 28663337. Bibcode: 2017PNAS..114.7194W.
- ↑ "LINE-1 elements in structural variation and disease". Annual Review of Genomics and Human Genetics 12 (1): 187–215. 2011. doi:10.1146/annurev-genom-082509-141802. PMID 21801021.
- ↑ "The NF1 gene contains hotspots for L1 endonuclease-dependent de novo insertion". PLOS Genetics 7 (11): e1002371. November 2011. doi:10.1371/journal.pgen.1002371. PMID 22125493.
- ↑ "Gonorrhea has picked up human DNA (and that's just the beginning)". National Geographic. 2011-02-16. https://www.nationalgeographic.com/science/phenomena/2011/02/16/gonorrhea-has-picked-up-human-dna-and-thats-just-the-beginning/.
- ↑ "Opportunity and means: horizontal gene transfer from the human host to a bacterial pathogen". mBio 2 (1): e00005-11. 2011. doi:10.1128/mBio.00005-11. PMID 21325040.
- ↑ "Unconventional translation of mammalian LINE-1 retrotransposons". Genes & Development 20 (2): 210–24. January 2006. doi:10.1101/gad.1380406. PMID 16418485.
- ↑ "The dicistronic RNA from the mouse LINE-1 retrotransposon contains an internal ribosome entry site upstream of each ORF: implications for retrotransposition". Nucleic Acids Research 34 (3): 853–64. 2006-01-01. doi:10.1093/nar/gkj490. PMID 16464823.
- ↑ "Binding of the ubiquitous nuclear transcription factor YY1 to a cis regulatory sequence in the human LINE-1 transposable element". Human Molecular Genetics 2 (10): 1697–702. October 1993. doi:10.1093/hmg/2.10.1697. PMID 8268924. https://zenodo.org/record/1234317.
- ↑ "Members of the SRY family regulate the human LINE retrotransposons". Nucleic Acids Research 28 (2): 411–5. January 2000. doi:10.1093/nar/28.2.411. PMID 10606637.
- ↑ "A YY1-binding site is required for accurate human LINE-1 transcription initiation". Nucleic Acids Research 32 (13): 3846–55. 2004-01-01. doi:10.1093/nar/gkh698. PMID 15272086.
- ↑ "An antisense promoter in mouse L1 retrotransposon open reading frame-1 initiates expression of diverse fusion transcripts and limits retrotransposition". Nucleic Acids Research 42 (7): 4546–62. April 2014. doi:10.1093/nar/gku091. PMID 24493738.
- ↑ "L1 antisense promoter drives tissue-specific transcription of human genes". Journal of Biomedicine & Biotechnology 2006 (1): 71753. 2006. doi:10.1155/JBB/2006/71753. PMID 16877819.
- ↑ "The ORF1 protein encoded by LINE-1: structure and function during L1 retrotransposition". Journal of Biomedicine & Biotechnology 2006 (1): 45621. 2006. doi:10.1155/jbb/2006/45621. PMID 16877816.
- ↑ Kaur, D; Agrahari, M; Singh, SS; Mandal, PK; Bhattacharya, A; Bhattacharya, S (March 2021). "Transcriptomic analysis of Entamoeba histolytica reveals domain-specific sense strand expression of LINE-encoded ORFs with massive antisense expression of RT domain.". Plasmid 114: 102560. doi:10.1016/j.plasmid.2021.102560. PMID 33482228.
- ↑ "Mobile DNA in cancer. Extensive transduction of nonrepetitive DNA mediated by L1 retrotransposition in cancer genomes". Science 345 (6196): 1251343. August 2014. doi:10.1126/science.1251343. PMID 25082706.
- ↑ "A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer". Journal of the National Cancer Institute 100 (23): 1734–8. December 2008. doi:10.1093/jnci/djn359. PMID 19033568.
- ↑ "LINE-1 hypomethylation during primary colon cancer progression". PLOS ONE 6 (4): e18884. April 2011. doi:10.1371/journal.pone.0018884. PMID 21533144. Bibcode: 2011PLoSO...618884S.
- ↑ "Quantification of LINE1 in circulating DNA as a molecular biomarker of breast cancer". Annals of the New York Academy of Sciences 1137 (1): 171–4. August 2008. doi:10.1196/annals.1448.011. PMID 18837943. Bibcode: 2008NYASA1137..171S.
- ↑ "Implications of LINE1 methylation for bladder cancer risk in women". Clinical Cancer Research 16 (5): 1682–9. March 2010. doi:10.1158/1078-0432.CCR-09-2983. PMID 20179218.
- ↑ "L1 retrotransposition in human neural progenitor cells". Nature 460 (7259): 1127–31. August 2009. doi:10.1038/nature08248. PMID 19657334. Bibcode: 2009Natur.460.1127C.
- ↑ "Mosaic copy number variation in human neurons". Science 342 (6158): 632–7. November 2013. doi:10.1126/science.1243472. PMID 24179226. Bibcode: 2013Sci...342..632M.
- ↑ 24.0 24.1 "Mobile DNA elements in the generation of diversity and complexity in the brain". Nature Reviews. Neuroscience 15 (8): 497–506. August 2014. doi:10.1038/nrn3730. PMID 25005482.
- ↑ "L1 retrotransposition in neurons is modulated by MeCP2". Nature 468 (7322): 443–6. November 2010. doi:10.1038/nature09544. PMID 21085180. Bibcode: 2010Natur.468..443M.
- ↑ "Lower LINE-1 methylation in first-episode schizophrenia patients with the history of childhood trauma". Epigenomics 7 (8): 1275–1285. 2015-12-01. doi:10.2217/epi.15.68. PMID 26212695.
- ↑ "Identification of LINE retrotransposons and long non-coding RNAs expressed in the octopus brain". BMC Biology 20 (1): 116. May 2022. doi:10.1186/s12915-022-01303-5. PMID 35581640.
- ↑ "DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration". Nature 471 (7338): 325–30. March 2011. doi:10.1038/nature09830. PMID 21297615. Bibcode: 2011Natur.471..325K.
- ↑ "Effects of L1 retrotransposon insertion on transcript processing, localization and accumulation: lessons from the retinal degeneration 7 mouse and implications for the genomic ecology of L1 elements". Human Molecular Genetics 15 (13): 2146–56. July 2006. doi:10.1093/hmg/ddl138. PMID 16723373.
- ↑ Wang, F., Chamani, I.J., Luo, D. et al. (2021). Inhibition of LINE-1 retrotransposition represses telomere reprogramming during mouse 2-cell embryo development. J Assist Reprod Genet https://doi.org/10.1007/s10815-021-02331-w
- ↑ Kohlrausch, F. B., Berteli, T. S., Wang, F., Navarro, P. A., & Keefe, D. L. (2021). Control of LINE-1 Expression Maintains Genome Integrity in Germline and Early Embryo Development. Reproductive Sciences, 1-13. PMID 33481218 doi:10.1007/s43032-021-00461-1
- ↑ "Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues". Proceedings of the National Academy of Sciences of the United States of America 118 (21): e2105968118. May 2021. doi:10.1073/pnas.2105968118. PMID 33958444. Bibcode: 2021PNAS..11805968Z.
- ↑ "No evidence of human genome integration of SARS-CoV-2 found by long-read DNA sequencing". Cell Reports 36 (7): 109530. August 2021. doi:10.1016/j.celrep.2021.109530. PMID 34380018.
- ↑ "No evidence of SARS-CoV-2 reverse transcription and integration as the origin of chimeric transcripts in patient tissues". Proceedings of the National Academy of Sciences of the United States of America 118 (33): e2109066118. August 2021. doi:10.1073/pnas.2109066118. PMID 34344759. Bibcode: 2021PNAS..11809066P.
- ↑ "Host-Virus Chimeric Events in SARS-CoV-2-Infected Cells Are Infrequent and Artifactual". Journal of Virology 95 (15): e0029421. July 2021. doi:10.1128/JVI.00294-21. PMID 33980601.
- ↑ "L1Base 2: more retrotransposition-active LINE-1s, more mammalian genomes". Nucleic Acids Research 45 (D1): D68–D73. January 2017. doi:10.1093/nar/gkw925. PMID 27924012.
Further reading
- "The Rhox gene cluster suppresses germline LINE1 transposition". Proceedings of the National Academy of Sciences of the United States of America 118 (23): e2024785118. June 2021. doi:10.1073/pnas.2024785118. PMID 34083437. Bibcode: 2021PNAS..11824785T.
- "RHOX10 drives mouse spermatogonial stem cell establishment through a transcription factor signaling cascade". Cell Reports 36 (3): 109423. July 2021. doi:10.1016/j.celrep.2021.109423. PMID 34289349.
- "Factors Regulating the Activity of LINE1 Retrotransposons". Genes 12 (10): 1562. September 2021. doi:10.3390/genes12101562. PMID 34680956.
- "LINE-1 vectors mediate recombinant antibody gene transfer by retrotransposition in Chinese hamster ovary cells". Biotechnology Journal 16 (7): e2000620. July 2021. doi:10.1002/biot.202000620. PMID 33938150.
- "LINE-1 activation after fertilization regulates global chromatin accessibility in the early mouse embryo". Nature Genetics 49 (10): 1502–1510. October 2017. doi:10.1038/ng.3945. PMID 28846101.
- "A comparative analysis of L1 retrotransposition activities in human genomes suggests an ongoing increase in L1 number despite an evolutionary trend towards lower activity". Mobile DNA 12 (1): 26. November 2021. doi:10.1186/s13100-021-00255-x. PMID 34782009.
- "LINE-1 retrotransposition in human embryonic stem cells". Human Molecular Genetics 16 (13): 1569–1577. July 2007. doi:10.1093/hmg/ddm105. PMID 17468180.
- "Homotypic clustering of L1 and B1/Alu repeats compartmentalizes the 3D genome". Cell Research 31 (6): 613–630. June 2021. doi:10.1038/s41422-020-00466-6. PMID 33514913.
 |
