Biology:Link domain
| Link domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
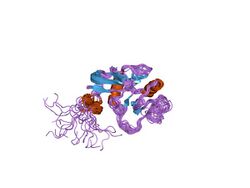 Structure of the hyaluronan-binding domain of human CD44 | |||||||||
| Identifiers | |||||||||
| Symbol | LINK | ||||||||
| Pfam | PF00193 | ||||||||
| Pfam clan | CL0056 | ||||||||
| InterPro | IPR000538 | ||||||||
| SMART | SM00445 | ||||||||
| PROSITE | PDOC00955 | ||||||||
| SCOP2 | 1o7b / SCOPe / SUPFAM | ||||||||
| CDD | cd01102 | ||||||||
| |||||||||
A Link domain or Link module, also known as Xlink domain (X for extracellular), is a protein domain that binds to hyaluronic acid.[1] It is important in blood cell migration and apoptosis.[2] The link domain is found in some extracellular proteins in vertebrates such as the hyalectans.[3] It appears to be involved in extracellular matrix assembly and stability, cell adhesion, and migration.[3][4]
Structure
The structure has been shown to consist of two alpha helices and two antiparallel beta sheets arranged around a large hydrophobic core similar to that of C-type lectin.[5] This domain contains four conserved cysteines involved in two disulphide bonds. The link domain has also been termed HABM (hyaluronic acid binding module)[4] and PTR (proteoglycan tandem repeat).[6]
Link domain proteins
Proteins which contain the link domain include:
- the hyalectans (a family of proteoglycans): aggrecan, brevican, neurocan and versican, which are expressed in the CNS;
- the cartilage link protein (LP), a proteoglycan that together with HA and aggrecan forms multimolecular aggregates;
- Tumour necrosis factor-inducible protein 6 (TSG-6), which may be involved in cell-cell and cell-matrix interactions during inflammation and tumourigenesis;
- CD44 antigen, the main cell surface receptor for HA.
See also
References
- ↑ "Link domain signature and profile". December 2004. http://prosite.expasy.org/cgi-bin/prosite/prosite-search-ac?PDOC00955.
- ↑ "Evidence for the heparin-binding ability of the ascidian Xlink domain and insight into the evolution of the Xlink domain in chordates.". J Mol Evol 71 (1): 51–9. 2010. doi:10.1007/s00239-010-9363-x. PMID 20582409. Bibcode: 2010JMolE..71...51Y.
- ↑ 3.0 3.1 Hynes, RO; Naba, A (21 September 2011). "Overview of the Matrisome--An Inventory of Extracellular Matrix Constituents and Functions". Cold Spring Harbor Perspectives in Biology 4 (1): a004903. doi:10.1101/cshperspect.a004903. PMID 21937732.
- ↑ 4.0 4.1 "Evolution of the hyaluronan-binding module of link protein". Biochem. J. 292 (3): 947–9. June 1993. doi:10.1042/bj2920947. PMID 8318021.
- ↑ "Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration". Cell 86 (5): 767–75. September 1996. doi:10.1016/S0092-8674(00)80151-8. PMID 8797823.
- ↑ "The protein fold of the hyaluronate-binding proteoglycan tandem repeat domain of link protein, aggrecan and CD44 is similar to that of the C-type lectin superfamily". FEBS Lett. 388 (2–3): 211–6. June 1996. doi:10.1016/0014-5793(96)00576-5. PMID 8690089.
 |
