Biology:Mitochondrial ROS
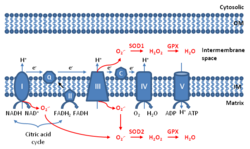
Mitochondrial ROS (mtROS or mROS) are reactive oxygen species (ROS) that are produced by mitochondria.[1][2][3] Generation of mitochondrial ROS mainly takes place at the electron transport chain located on the inner mitochondrial membrane during the process of oxidative phosphorylation. Leakage of electrons at complex I and complex III from electron transport chains leads to partial reduction of oxygen to form superoxide. Subsequently, superoxide is quickly dismutated to hydrogen peroxide by two dismutases including superoxide dismutase 2 (SOD2) in mitochondrial matrix and superoxide dismutase 1 (SOD1) in mitochondrial intermembrane space. Collectively, both superoxide and hydrogen peroxide generated in this process are considered as mitochondrial ROS.[1]
Once thought as merely the by-products of cellular metabolism, mitochondrial ROS are increasingly viewed as important signaling molecules,[4] whose levels of generation at 11 currently-identified sites vary depending on cellular energy supply and demand.[5][6] At low levels, mitochondrial ROS are considered to be important for metabolic adaptation as seen in hypoxia.[1] Mitochondrial ROS, stimulated by danger signals such as lysophosphatidylcholine and Toll-like receptor 4 and Toll-like receptor 2 bacterial ligands lipopolysaccharide (LPS) and lipopeptides, are involved in regulating inflammatory response.[7][8] Finally, high levels of mitochondrial ROS activate apoptosis/autophagy pathways capable of inducing cell death.[9]
COVID-19
Monocytes/macrophages are the most enriched immune cell types in the lungs of COVID-19 patients and appear to have a central role in the pathogenicity of the disease. These cells adapt their metabolism upon infection and become highly glycolytic, which facilitates SARS-CoV-2 replication. The infection triggers mitochondrial ROS production, which induces stabilization of hypoxia-inducible factor-1α (HIF1A) and consequently promotes glycolysis. HIF1A-induced changes in monocyte metabolism by SARS-CoV-2 infection directly inhibit T cell response and reduce epithelial cell survival. Targeting mitochondrial ROS may have great therapeutic potential for the development of novel drugs to treat patients with coronavirus.[10]
Aging
Mitochondrial ROS can promote cellular senescence and aging phenotypes in the skin of mice.[11] Ordinarily mitochondrial SOD2 protects against mitochondrial ROS. Epidermal cells in mutant mice with a genetic SOD2 deficiency undergo cellular senescence, nuclear DNA damage, and irreversible arrest of proliferation in a portion of their keratinocytes.[11][12]
Mutant mice with a conditional deficiency for mitochondrial SOD2 in connective tissue have an accelerated aging phenotype.[13] This aging phenotype includes weight loss, skin atrophy, kyphosis (curvature of the spine), osteoporosis, muscle degeneration and reduced life span.
DNA damage
Mitochondrial ROS attack DNA readily, generating a variety of DNA damages such as oxidized bases and strand breaks. The major mechanism that cells use to repair oxidized bases such as 8-hydroxyguanine, formamidopyrimidine and 5-hydroxyuracil is base excision repair (BER).[14] BER occurs in both the cell nucleus and in mitochondria.
References
- ↑ 1.0 1.1 1.2 "Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers". J Hematol Oncol. 6 (19): 19. February 2013. doi:10.1186/1756-8722-6-19. PMID 23442817.
- ↑ Reichart, Gesine (October 30, 2018). "Mitochondrial complex IV mutation increases ROS production and reduces lifespan in aged mice.". Acta Physiologica 225 (4): e13214. doi:10.1111/apha.13214. PMID 30376218.
- ↑ "Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells.". Can J Physiol Pharmacol 95 (3): 247–252. March 2017. doi:10.1139/cjpp-2016-0515. PMID 27925481.
- ↑ Trewin, Adam J; Bahr, Laura L; Almast, Anmol; Berry, Brandon J; Wei, Alicia Y; Foster, Thomas H; Wojtovich, Andrew P (2019-03-19). "Mitochondrial ROS generated at the complex-II matrix or intermembrane space microdomain have distinct effects on redox signaling and stress sensitivity in C. elegans.". Antioxidants & Redox Signaling 31 (9): 594–607. doi:10.1089/ars.2018.7681. ISSN 1523-0864. PMID 30887829.
- ↑ Trewin, Adam J.; Parker, Lewan; Shaw, Christopher S.; Hiam, Danielle S.; Garnham, Andrew; Levinger, Itamar; McConell, Glenn K.; Stepto, Nigel K. (November 2018). "Acute HIIE elicits similar changes in human skeletal muscle mitochondrial H 2 O 2 release, respiration, and cell signaling as endurance exercise even with less work". American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 315 (5): R1003–R1016. doi:10.1152/ajpregu.00096.2018. ISSN 0363-6119. PMID 30183338.
- ↑ Goncalves, Renata L. S.; Quinlan, Casey L.; Perevoshchikova, Irina V.; Hey-Mogensen, Martin; Brand, Martin D. (2015-01-02). "Sites of Superoxide and Hydrogen Peroxide Production by Muscle Mitochondria Assessed ex Vivo under Conditions Mimicking Rest and Exercise". Journal of Biological Chemistry 290 (1): 209–227. doi:10.1074/jbc.M114.619072. ISSN 0021-9258. PMID 25389297.
- ↑ "Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation". Arteriosclerosis, Thrombosis, and Vascular Biology 36 (6): 1090–100. April 2016. doi:10.1161/ATVBAHA.115.306964. PMID 27127201.
- ↑ "TLR signalling augments macrophage bactericidal activity through mitochondrial ROS.". Nature 472 (7344): 476–480. April 2011. doi:10.1038/nature09973. PMID 21525932. Bibcode: 2011Natur.472..476W.
- ↑ Finkel T (February 2012). "Signal transduction by mitochondrial oxidants". J Biol Chem 287 (7): 4434–40. doi:10.1074/jbc.R111.271999. PMID 21832045.
- ↑ Cavounidis A, Mann EH (June 2020). "SARS-CoV-2 has a sweet tooth". Nature Reviews Immunology 20 (8): 460. doi:10.2139/ssrn.3606770. PMID 32533110.
- ↑ 11.0 11.1 "Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin". Aging (Albany NY) 4 (1): 3–12. January 2012. doi:10.18632/aging.100423. PMID 22278880.
- ↑ "Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells". Proc. Natl. Acad. Sci. U.S.A. 112 (33): 10407–12. August 2015. doi:10.1073/pnas.1505675112. PMID 26240345. Bibcode: 2015PNAS..11210407V.
- ↑ "Accelerated aging phenotype in mice with conditional deficiency for mitochondrial superoxide dismutase in the connective tissue". Aging Cell 10 (2): 239–54. April 2011. doi:10.1111/j.1474-9726.2010.00658.x. PMID 21108731.
- ↑ "Base excision repair of oxidative DNA damage and association with cancer and aging". Carcinogenesis 30 (1): 2–10. January 2009. doi:10.1093/carcin/bgn250. PMID 18978338.
 |