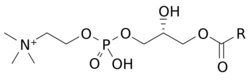
Chemistry:Lysophosphatidylcholine
Lysophosphatidylcholines (LPC, lysoPC), also called lysolecithins, are a class of chemical compounds which are derived from phosphatidylcholines.[1]
Overview
Lysophosphatidylcholines are produced within cells mainly by the enzyme phospholipase A2, which removes one of the fatty acid groups from phosphatidylcholine to produce LPC.[2] Among other properties, they activate endothelial cells during early atherosclerosis.[3][4] LPC also acts as a find-me signal, released by apoptotic cells to recruit phagocytes, which then phagocytose the apoptotic cells [5] Moreover, LPCs can be used in the lab to cause demyelination of brain slices, to mimic the effects of demyelinating diseases such as multiple sclerosis. Further, they are known to stimulate phagocytosis of the myelin sheath and can change the surface properties of erythrocytes.[6] LPC-induced demyelination is thought to occur through the actions of recruited macrophages and microglia which phagocytose nearby myelin. Invading T cells are also thought to mediate this process. Bacteria such as Legionella pneumophila utilize phospholipase A2 end-products (fatty acids and lysophospholipids) to cause host cell (macrophage) apoptosis through cytochrome C release.
LPCs are present as minor phospholipids in the cell membrane (≤ 3%) and in the blood plasma (8–12%).[6] Since LPCs are quickly metabolized by lysophospholipase and LPC-acyltransferase, they last only shortly in vivo. By replacing the acyl-group within the LPC with an alkyl-group, alkyl-lysophospholipids (ALP) were synthesized. These LPC analogues are metabolically stable, and several such as edelfosine, miltefosine and perifosine are under research and development as drugs against cancer and other diseases.[6][7] Lysophosphatidylcholine processing has been discovered to be an essential component of normal human brain development: those born with genes that prevent adequate uptake suffer from lethal microcephaly.[8] MFSD2a has been shown to transport LPC-bound polyunsaturated fatty acids, including DHA and EPA, across the blood-brain and blood-retinal barriers.[9] [10]
LPCs occur in many foods naturally. In Starch: Chemistry and Technology third edition on page 592, the authors state that "lysophosphatidylcholine makes up about 70% of the lipids in oat starch".[11]
The anti-cancer abilities of synthetic LPC variants are special since they do not target the cell DNA but insert into the plasma membrane and cause apoptosis through influencing several signal pathways. Therefore, their effects are independent of the proliferation state of the tumor cell.[12]
Industrial Applications of Enzymes Producing Lysophosphatidylcholine
FoodPro LysoMaxa Oil is an FDA approved commercialized PLA2 enzyme preparation utilized for the degumming of vegetable oils in large-scale productions to increase yield. Variants of lysophosphatidylcholine are the main products of this enzyme. [13] Lysophosphatidylcholine has been studied as an immune activator for differentiating monocytes to mature dendritic cells.[14] Lysophosphatidylcholine present in blood amplifies microbial TLR ligands induced inflammatory responses from human cells like intestinal epithelial cells and macrophages/monocytes.[15] This has an implication in sepsis induced by microbes.
Composition in Foods
Lysophosphatidylcholine accounts for 4.6% of phospholipids found in coconut oil, which make up 0.2% of lipids in coconut oil. This is compared to vegetable oils, which may contain 2-3% phospholipids.[16]
Lysophosphatidylcholine and Atherosclerosis
Intima-media thickness, which is positively correlated with reduced blood flow, was studied in young smokers. Evidence pointed towards smoking as a major risk factor for increased levels of PLA2, due to tobacco smoke's impact on oxidation of retained LDL particles in the intima of a carotid artery.[17] which may have a detrimental impact on overall health.
See also
References
- ↑ "Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation.". The Journal of Biological Chemistry 293 (28): 11033–11045. May 2018. doi:10.1074/jbc.RA118.002752. PMID 29769317.
- ↑ Phosphatidylcholine and related lipids , lipidlibrary.co.uk
- ↑ "Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation". Arteriosclerosis, Thrombosis, and Vascular Biology 36 (6): 1090–100. April 2016. doi:10.1161/ATVBAHA.115.306964. PMID 27127201.
- ↑ "IL-35 (Interleukin-35) Suppresses Endothelial Cell Activation by Inhibiting Mitochondrial Reactive Oxygen Species-Mediated Site-Specific Acetylation of H3K14 (Histone 3 Lysine 14).". Arteriosclerosis, Thrombosis, and Vascular Biology 38 (3): 599–609. March 2018. doi:10.1161/ATVBAHA.117.310626. PMID 29371247.
- ↑ Lauber, K; Bohn, E; Kröber, SM; Xiao, Y (2003). "Apoptotic Cells Induce Migration of Phagocytes via Caspase-3-Mediated Release of a Lipid Attraction Signal". Cell 113 (6): 717–730. doi:10.1016/S0092-8674(03)00422-7. PMID 12809603.
- ↑ 6.0 6.1 6.2 Munder, PG; Modolell M; Andreesen R; Weltzien HU; Westphal O (1979). "Lysophosphatidylcholine (Lysolecithin) and its Synthetic Analogues. Immunemodulating and Other Biologic Effects". Springer Seminars in Immunopathology 203 (2): 187–203. doi:10.1007/bf01891668.
- ↑ Houlihan, W; Lohmeyer M; Workman P; Cheon SH (1995). "Phospholipid antitumor agents.". Medicinal Research Reviews 15 (3): 157–223. doi:10.1002/med.2610150302. PMID 7658750.
- ↑ Guemez-Gamboa, Alicia; N Nguyen; Hongbo Yan (2015). "Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome.". Nature Genetics 47 (7): 809–813. doi:10.1038/ng.3311. PMID 26005868.
- ↑ Nguyen, LN; Ma, D; Shui, G; Wong, P; Cazenave-Gassiot, A; Zhang, X; Wenk, MR; Goh, ELK et al. (2014-05-22). "Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid". Nature 509 (7501): 503–506. doi:10.1038/nature13241. PMID 24828044. Bibcode: 2014Natur.509..503N. https://doi.org/10.1038/nature13241.
- ↑ Wong, BH; Chan, JP; Cazenave-Gassiot, A; Poh, RW; Foo, JC; Galam, DLA; Gosh, S; Nguyen, LN et al. (2016). "Mfsd2a Is a Transporter for the Essential ω-3 Fatty Acid Docosahexaenoic Acid (DHA) in Eye and Is Important for Photoreceptor Cell Development". J Biol Chem 291 (20): 10501–14. doi:10.1074/jbc.M116.721340. PMID 27008858.
- ↑ Bemiller, James N.; Whistler, Roy L. (2009-04-06). Starch. Academic Press. ISBN 9780080926551. https://books.google.com/books?id=Anbz_whRM2YC&q=starch+lysophosphatidylcholine&pg=PA592.
- ↑ van Blitterswijk, W; Verheij M (2008). "Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects.". Current Pharmaceutical Design 14 (21): 2061–74. doi:10.2174/138161208785294636. PMID 18691116.
- ↑ Michael Eskin, N. A.; Shahidi, Fereidoon (2012-10-08). Biochemistry of Foods. Academic Press. ISBN 9780080918099. https://books.google.com/books?id=cl3Pq5YzPxgC&q=biochemistry+of+foods.
- ↑ "Patent US20110135684 - Use of L-alpha-lysophosphatidylcholine to obtain the differentiation of...". google.com.mx. http://www.google.com.mx/patents/US20110135684.
- ↑ Sharma, Naveen; Akhade, Ajay Suresh; Ismaeel, Sana; Qadri, Ayub (2020). "Serum-borne lipids amplify TLR-activated inflammatory responses". Journal of Leukocyte Biology 109 (4): 821–831. doi:10.1002/JLB.3AB0720-241RR. PMID 32717772.
- ↑ "Rahman's page 12 chart". Archived from the original on 2014-03-28. https://web.archive.org/web/20140328224401/http://fos.ubd.edu.bn/sites/default/files/2000-Paper2.pdf.
- ↑ Fratta Pasini, A; Stranieri, C; Pasini, A; Vallerio, P; Mozzini, C; Solani, E; Cominacini, M; Cominacini, L et al. (2013). "Lysophosphatidylcholine and Carotid Intima-Media Thickness in Young Smokers: A Role for Oxidized LDL-Induced Expression of PBMC Lipoprotein-Associated Phospholipase A2?". PLOS ONE 8 (12): e83092. doi:10.1371/journal.pone.0083092. PMID 24358251. Bibcode: 2013PLoSO...883092F.
 |