Biology:Mixotroph
A mixotroph is an organism that uses a mix of different sources of energy and carbon, instead of having a single trophic mode. Mixotrophs are situated somewhere on the continuum from complete autotrophy to complete heterotrophy. It is estimated that mixotrophs comprise more than half of all microscopic plankton.[1] There are two types of eukaryotic mixotrophs. There are those with their own chloroplasts – including those with endosymbionts providing the chloroplasts. And there are those that acquire them through kleptoplasty, or through symbiotic associations with prey, or through 'enslavement' of the prey's organelles.[2]
Possible combinations include photo- and chemotrophy, besides litho- and organotrophy, the latter including osmotrophy, phagotrophy and myzocytosis. Mixotrophs can be either eukaryotic or prokaryotic.[3] Mixotrophs can take advantage of different environmental conditions.[4]
A given trophic mode of a mixotroph organism is called obligate when it is indispensable for its growth and maintenance; a trophic mode is facultative when used as a supplemental source.[3] Some organisms have incomplete Calvin cycles, so that they are incapable of fixing carbon dioxide and must use organic carbon sources.
Obligate or facultative
Organisms may employ mixotrophy obligately or facultatively.
- Obligate mixotrophy: To support growth and maintenance, an organism must utilize both heterotrophic and autotrophic means.
- Obligate autotrophy with facultative heterotrophy: Autotrophy alone is sufficient for growth and maintenance, but heterotrophy may be used as a supplementary strategy when autotrophic energy is not enough, for example, when light intensity is low.
- Facultative autotrophy with obligate heterotrophy: Heterotrophy is sufficient for growth and maintenance, but autotrophy may be used to supplement, for example, when prey availability is very low.
- Facultative mixotrophy: Maintenance and growth may be obtained by heterotrophic or autotrophic means alone, and mixotrophy is used only when necessary.[5]
Plants

Amongst plants, mixotrophy classically applies to carnivorous, hemi-parasitic and myco-heterotrophic species. However, this characterisation as mixotrophic could be extended to a higher number of clades as research demonstrates that organic forms of nitrogen and phosphorus—such as DNA, proteins, amino-acids or carbohydrates—are also part of the nutrient supplies of a number of plant species.[6]
Mycoheterotrophic plants form symbiotic relationships with mycorrhizal fungi, which provide them with organic carbon and nutrients from nearby photosynthetic plants or soil. They often lack chlorophyll or have reduced photosynthetic capacity. An example is Indian pipe, a white, non-photosynthetic plant that relies heavily on fungal networks for nutrients. Pinesap also taps into fungal networks for sustenance, similar to Indian pipe. Certain orchids, such as Corallorhiza, depend on fungi for carbon and nutrients while developing photosynthetic capabilities (especially in their early stages).


Carnivorous plants are plants that derive some or most of their nutrients from trapping and consuming animals[7] or protozoans, typically insects and other arthropods, and occasionally small mammals and birds. They have adapted to grow in waterlogged sunny places where the soil is thin or poor in nutrients, especially nitrogen, such as acidic bogs.[8]
Hemiparasitic plants are partially parasitic, attaching to the roots or stems of host plants to extract water, nutrients, or organic compounds while still performing photosynthesis. Examples are mistletoe (absorbs water and nutrients from host trees but also photosynthesizes), Indian paintbrush (connects to the roots of other plants for nutrients while maintaining photosynthetic leaves), and Yellow rattle (a root parasite that supplements its nutrition by tapping into host plants).
Some epiphytic plants, which are plants that grow on other plants, absorb organic matter, such as decaying debris or animal waste, through specialized structures while still photosynthesizing. For example, some bromeliads have tank-like leaf structures that collect water and organic debris, absorbing nutrients through their leaves. Also, some epiphytic orchids absorb nutrients from organic matter caught in their aerial roots.
Some plants incorporate algae or cyanobacteria, which provide photosynthetically derived carbon, while the plant also absorbs external nutrients. For example, Azolla filiculoides, is a floating fern that hosts the nitrogen-fixing cyanobacteria Anabaena in its leaves, supplementing nutrient intake while photosynthesizing. This has led to the plant being dubbed a "super-plant", as it can readily colonise areas of freshwater, and grow at great speed - doubling its biomass in as little as 1.9 days.[9]
Animals
Mixotrophy is less common among animals than among plants and microbes, but there are many examples of mixotrophic invertebrates and at least one example of a mixotrophic vertebrate.
- The spotted salamander, Ambystoma maculatum, also hosts microalgae within its cells. Its embryos have been found to have symbiotic algae living inside them,[10] the only known example of vertebrate cells hosting an endosymbiont microbe (unless mitochondria is considered).[11][12]
- Zoochlorella is a nomen rejiciendum for a genus of green algae assigned to Chlorella.[13] The term zoochlorella (plural zoochlorellae) is sometimes used to refer to any green algae that lives symbiotically within the body of a freshwater or marine invertebrate or protozoan.
- Reef-building corals (Scleractinia), like many other cnidarians (e.g. jellyfish, anemones), host endosymbiotic microalgae within their cells, thus making them mixotrophs.
- The Oriental hornet, Vespa orientalis, can obtain energy from sunlight absorbed by its cuticle.[14] It thus contrasts with the other animals listed here, which are mixotrophic with the help of endosymbionts.
- The Leaf sheep, Costasiella kuroshimae, retains chloroplasts from algae it consumes so it can supplement its diet with photosynthesis via kleptoplasty
-
Zooxanthellae is a photosynthetic algae that lives inside hosts like coral.
-
Anthopleura xanthogrammica gains its green colour from Zoochlorella.
-
The spotted jelly, a mixotrophic jellyfish, lives in trophic mutualism with zooxanthella, a unicellular organism capable of photosynthesis.[15]
Microorganisms
Bacteria and archaea
- Paracoccus pantotrophus is a bacterium that can live chemoorganoheterotrophically, whereby many organic compounds can be metabolized. Also, a facultative chemolithoautotrophic metabolism is possible, as seen in colorless sulfur bacteria (some Thiobacillus), whereby sulfur compounds such as hydrogen sulfide, elemental sulfur, or thiosulfate are oxidized to sulfate. The sulfur compounds serve as electron donors and are consumed to produce ATP. The carbon source for these organisms can be carbon dioxide (autotrophy) or organic carbon (heterotrophy).[16][17][18]
Organoheterotrophy can occur under aerobic or under anaerobic conditions; lithoautotrophy takes place aerobically.[19][20]
Classifying protists

DIN = dissolved inorganic nutrients
Several categorization schemes have been suggested to characterize sub-domains within mixotrophy.
Phototrophy verses phagotrophy
Consider the example of a marine protist with heterotrophic and photosynthetic capabilities: In the breakdown put forward by Jones,[22] there are four mixotrophic groups based on relative roles of phagotrophy and phototrophy.
- A: Heterotrophy (phagotrophy) is the norm, and phototrophy is only used when prey concentrations are limiting.
- B: Phototrophy is the dominant strategy, and phagotrophy is employed as a supplement when light is limiting.
- C: Phototrophy results in substances for both growth and ingestion; phagotrophy is employed when light is limiting.
- D: Phototrophy is most common nutrition type, phagotrophy only used during prolonged dark periods, when light is extremely limiting.
By efficiency
An alternative scheme by Stoeker[21] also takes into account the role of nutrients and growth factors, and includes mixotrophs that have a photosynthetic symbiont or who retain chloroplasts from their prey. This scheme characterizes mixotrophs by their efficiency.
- Type 1: "Ideal mixotrophs" that use prey and sunlight equally well
- Type 2: Supplement phototrophic activity with food consumption
- Type 3: Primarily heterotrophic, use phototrophic activity during times of very low prey abundance.[24]
Constitutive mixotrophs
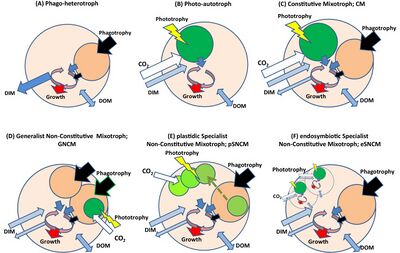
Another scheme, proposed by Mitra et al., specifically classifies marine planktonic mixotrophs so that mixotrophy can be included in ecosystem modeling.[23] This scheme classified organisms as:
- Constitutive mixotrophs (CMs): phagotrophic organisms that are inherently able also to photosynthesize
- Non-constitutive mixotrophs (NCMs): phagotrophic organisms that must ingest prey to attain the ability to photosynthesize. NCMs are further broken down into:
- Specific non-constitutive mixotrophs (SNCMs), which only gain the ability to photosynthesize from a specific prey item (either by retaining plastids only in kleptoplastidy or by retaining whole prey cells in endosymbiosis)
- General non-constitutive mixotrophs (GNCM), which can gain the ability to photosynthesize from a variety of prey items
(A) phagotrophic (no phototrophy); (B) phototrophic (no phagotrophy); (C) constitutive mixotroph, with innate capacity for phototrophy; (D) generalist non-constitutive mixotroph acquiring photosystems from different phototrophic prey; (E) specialist non-constitutive mixotroph acquiring plastids from a specific prey type; (F) specialist non-constitutive mixotroph acquiring photosystems from endosymbionts. DIM = dissolved inorganic material (ammonium, phosphate etc.). DOM = dissolved organic material
-
Acantharian radiolarian hosts Phaeocystis symbionts.
-
White Phaeocystis algal foam washing up on a beach
-
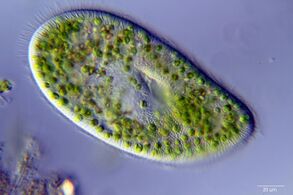
A single-celled ciliate with green zoochlorellae living inside endosymbiotically
-
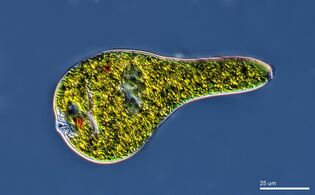
Euglena mutabilis, a photosynthetic flagellate
-
Euglenoid
-
Fluorescent micrograph of an acantharian with Phaeocystis symbionts fluorescing red (chlorophyll)
Marine food webs

Mixotrophs are especially common in marine environments, where the levels of energy from the sun and nutrients in the water can vary greatly. For example, in nutrient-poor (oligotrophic) waters, mixotrophic phytoplankton supplement their diet by consuming bacteria.[25][26]
The effects of mixotrophy on organic and inorganic carbon pools introduce a metabolic plasticity which blurs the lines between producers and consumers.[27] Prior to the discovery of mixotrophs, it was thought that only organisms with chloroplasts were capable of photosynthesis and vice versa. This additional functional group of plankton, capable of both phototrophy and phagotrophy, provides a further boost in the biomass and energy transfer to higher trophic levels.[28]

See also
Notes
- ↑ Mitra, Aditee (2022-11-03). "Uncovered: the mysterious killer triffids that dominate life in our oceans". https://theconversation.com/uncovered-the-mysterious-killer-triffids-that-dominate-life-in-our-oceans-67387.
- ↑ Leles S G et al, (2017). Oceanic protists with different forms of acquired phototrophy display contrasting biogeographies and abundance, Proceedings of the Royal Society B: Biological Sciences.
- ↑ 3.0 3.1 Eiler A (December 2006). "Evidence for the Ubiquity of Mixotrophic Bacteria in the Upper Ocean: Implications and Consequences". Appl Environ Microbiol 72 (12): 7431–7. doi:10.1128/AEM.01559-06. PMID 17028233. Bibcode: 2006ApEnM..72.7431E.
- ↑ "The mixotroph Ochromonas tuberculata may invade and suppress specialist phago- and phototroph plankton communities depending on nutrient conditions". Oecologia 148 (4): 692–701. July 2006. doi:10.1007/s00442-006-0413-4. PMID 16568278. Bibcode: 2006Oecol.148..692K.
- ↑ Schoonhoven, Erwin (January 19, 2000). "Ecophysiology of Mixotrophs". Thesis. http://www.bio.vu.nl/thb/education/Scho2000.pdf.
- ↑ Schmidt, Susanne; John A. Raven; Chanyarat Paungfoo-Lonhienne (2013). "The mixotrophic nature of photosynthetic plants". Functional Plant Biology 40 (5): 425–438. doi:10.1071/FP13061. ISSN 1445-4408. PMID 32481119. Bibcode: 2013FunPB..40..425S.
- ↑ "Carnivorous Plants - Plant Biology". https://plantbiology.siu.edu/facilities/plant-biology-facilities/greenhouse/topics/carnivorous.php.
- ↑ Insectivorous Plants. London: John Murray. 1875. http://darwin-online.org.uk/EditorialIntroductions/Freeman_InsectivorousPlants.html. Retrieved 14 March 2022.
- ↑ Iwao Watanabe, Nilda S.Berja (1983). "The growth of four species of Azolla as affected by temperature". Aquatic Botany 15 (2): 175–185. doi:10.1016/0304-3770(83)90027-X. Bibcode: 1983AqBot..15..175W.
- ↑ Petherick, Anna (2010-07-30). "A solar salamander" (in en). Nature. doi:10.1038/news.2010.384. ISSN 0028-0836. http://www.nature.com/articles/news.2010.384.
- ↑ Frazer, Jennifer (May 18, 2018). "Algae Living inside Salamanders Aren't Happy about the Situation". Scientific American Blog Network. https://blogs.scientificamerican.com/artful-amoeba/algae-living-inside-salamanders-arent-happy-about-the-situation/.
- ↑ Burns, John A; Zhang, Huanjia; Hill, Elizabeth; Kim, Eunsoo; Kerney, Ryan (2 May 2017). "Transcriptome analysis illuminates the nature of the intracellular interaction in a vertebrate-algal symbiosis". eLife 6. doi:10.7554/eLife.22054. PMID 28462779.
- ↑ Compère, Pierre (November 1999). "Report of the Committee for Algae: 6". Taxon 48 (1): 135–136.
- ↑ Plotkin, Hod, Zaban (2010). "Solar energy harvesting in the epicuticle of the oriental hornet (Vespa orientalis)". Naturwissenschaften 97 (12): 1067–1076. doi:10.1007/s00114-010-0728-1. PMID 21052618. Bibcode: 2010NW.....97.1067P.
- ↑ Djeghri, Nicolas; Pondaven, Philippe; Stibor, Herwig; Dawson, Michael N. (2019). "Review of the diversity, traits, and ecology of zooxanthellate jellyfishes". Marine Biology 166 (11): 147. doi:10.1007/s00227-019-3581-6. Bibcode: 2019MarBi.166..147D. https://archimer.ifremer.fr/doc/00604/71661/70246.pdf.
- ↑ Libes, Susan M. (2009). Introduction to marine biogeochemistry (2 ed.). Academic Press. p. 192. ISBN 978-0-7637-5345-0. https://books.google.com/books?id=KVZJUw4nORgC&q=chemolithoheterotrophs+inorganic&pg=PA192.
- ↑ Dworkin, Martin (2006). The Prokaryotes: Ecophysiology and biochemistry. 2 (3rd ed.). Springer. p. 988. ISBN 978-0-387-25492-0. https://books.google.com/books?id=uleTr2jKzJMC&q=chemolithoheterotroph+chemoorganoheterotroph&pg=PA988.
- ↑ Lengeler, Joseph W.; Drews, Gerhart; Schlegel, Hans Günter (1999). Biology of the Prokaryotes. Georg Thieme Verlag. p. 238. ISBN 978-3-13-108411-8. https://books.google.com/books?id=MiwpFtTdmjQC&q=chemolithoheterotroph+sulfur+bacteria&pg=PA238.
- ↑ "Identification and Characterization of Transposable Elements of Paracoccus pantotrophus". J Bacteriol 185 (13): 3753–63. July 2003. doi:10.1128/JB.185.13.3753-3763.2003. PMID 12813068.
- ↑ Friedrich, Cornelius G. (2007). "Redox Control of Chemotrophic Sulfur Oxidation of Paracoccus pantotrophus". Microbial Sulfur Metabolism. Springer. pp. 139–150. http://www.springerlink.com/content/x412771504738714/. PDF[|permanent dead link|dead link}}]
- ↑ 21.0 21.1 Stoecker, Diane K. (1998). "Conceptual models of mixotrophy in planktonic protists and some ecological and evolutionary implications". European Journal of Protistology 34 (3): 281–290. doi:10.1016/S0932-4739(98)80055-2.
- ↑ 22.0 22.1 Jones, Harriet (1997). "A classification of mixotrophic protists based on their behaviour". Freshwater Biology 37 (1): 35–43. doi:10.1046/j.1365-2427.1997.00138.x. Bibcode: 1997FrBio..37...35J.
- ↑ 23.0 23.1 23.2 23.3 Mitra, Aditee; Flynn, Kevin J.; Tillmann, Urban; Raven, John A.; Caron, David; Stoecker, Diane K.; Not, Fabrice; Hansen, Per J. et al. (2016). "Defining Planktonic Protist Functional Groups on Mechanisms for Energy and Nutrient Acquisition: Incorporation of Diverse Mixotrophic Strategies". Protist 167 (2): 106–120. doi:10.1016/j.protis.2016.01.003. PMID 26927496. 50px Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
- ↑ Tarangkoon, Woraporn (29 April 2010). "Mixtrophic Protists among Marine Ciliates and Dinoflagellates: Distribution, Physiology and Ecology". Thesis. http://www.bi.ku.dk/bibliotek/phd/Woraporn%20Tarangkoon.pdf.
- ↑ Schenone, Luca; Aarons, Zoe S.; García-Martínez, Minerva; Happe, Anika; Redoglio, Andrea (2024-11-25). "Mixotrophic protists and ecological stoichiometry: connecting homeostasis and nutrient limitation from organisms to communities". Frontiers in Ecology and Evolution 12. doi:10.3389/fevo.2024.1505037. ISSN 2296-701X. Bibcode: 2024FrEEv..1205037S.
- ↑ Wilken, Susanne; Verspagen, Jolanda M. H.; Naus-Wiezer, Suzanne; Van Donk, Ellen; Huisman, Jef (2014). "Biological control of toxic cyanobacteria by mixotrophic predators: an experimental test of intraguild predation theory". Ecological Applications 24 (5): 1235–1249. doi:10.1890/13-0218.1. ISSN 1051-0761. PMID 25154110. Bibcode: 2014EcoAp..24.1235W. https://pure.knaw.nl/ws/files/463990/5548_WilkenPreprint.pdf. Retrieved 2025-05-19.
- ↑ Worden, Alexandra Z.; Follows, Michael J.; Giovannoni, Stephen J.; Wilken, Susanne; Zimmerman, Amy E.; Keeling, Patrick J. (2015-02-13). "Rethinking the marine carbon cycle: Factoring in the multifarious lifestyles of microbes". Science 347 (6223). doi:10.1126/science.1257594. ISSN 0036-8075.
- ↑ Ward, Ben A.; Follows, Michael J. (2016-03-15). "Marine mixotrophy increases trophic transfer efficiency, mean organism size, and vertical carbon flux". Proceedings of the National Academy of Sciences 113 (11): 2958–2963. doi:10.1073/pnas.1517118113. ISSN 0027-8424. PMID 26831076. PMC 4801304. Bibcode: 2016PNAS..113.2958W. https://www.pnas.org/content/pnas/113/11/2958.full.pdf. Retrieved 2025-05-19..
- ↑ Stoecker, Diane K.; Lavrentyev, Peter J. (2018-08-22). "Mixotrophic Plankton in the Polar Seas: A Pan-Arctic Review". Frontiers in Marine Science 5: 292. doi:10.3389/fmars.2018.00292. ISSN 2296-7745. Bibcode: 2018FrMaS...5..292S.
External links
- "When do mixotrophs specialize? Adaptive dynamics theory applied to a dynamic energy budget model". Math Biosci 193 (2): 159–82. February 2005. doi:10.1016/j.mbs.2004.06.010. PMID 15748728.
- Sanders, Robert W. Mixotrophic Nutrition of Phytoplankton: Venus Fly Traps of the microbial world . Temple University.
 |