Biology:Molybdenum in biology
Molybdenum is an essential element in most organisms.[1] It is most notably present in nitrogenase[2] which is an essential part of nitrogen fixation.[3][4]
Mo-containing enzymes
Molybdenum is an essential element in most organisms; a 2008 research paper speculated that a scarcity of molybdenum in the Earth's early oceans may have strongly influenced the evolution of eukaryotic life (which includes all plants and animals).[1]
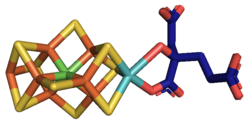
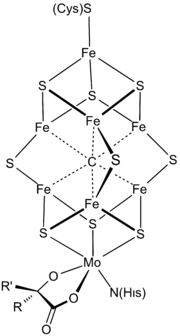
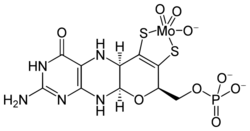
At least 50 molybdenum-containing enzymes have been identified, mostly in bacteria.[5][6] Those enzymes include aldehyde oxidase, sulfite oxidase and xanthine oxidase.[7] With one exception, Mo in proteins is bound by molybdopterin to give the molybdenum cofactor. The only known exception is nitrogenase, which uses the FeMoco cofactor, which has the formula Fe7MoS9C.[8]
In terms of function, molybdoenzymes catalyze the oxidation and sometimes reduction of certain small molecules in the process of regulating nitrogen, sulfur, and carbon.[9] In some animals, and in humans, the oxidation of xanthine to uric acid, a process of purine catabolism, is catalyzed by xanthine oxidase, a molybdenum-containing enzyme. The activity of xanthine oxidase is directly proportional to the amount of molybdenum in the body. An extremely high concentration of molybdenum reverses the trend and can inhibit purine catabolism and other processes. Molybdenum concentration also affects protein synthesis, metabolism, and growth.[10]
Mo is a component in most nitrogenases. Among molybdoenzymes, nitrogenases are unique in lacking the molybdopterin.[11][12] Nitrogenases catalyze the production of ammonia from atmospheric nitrogen:
- [math]\displaystyle{ \mathrm{N_2 + 8 \ H^+ + 8 \ e^- + 16 \ ATP + 16 \ H_2O \longrightarrow 2 \ NH_3 + H_2 + 16 \ ADP + 16 \ P_i} }[/math]
The biosynthesis of the FeMoco active site is highly complex.[13]
Molybdate is transported in the body as MoO42−.[10]
Human metabolism and deficiency
Molybdenum is an essential trace dietary element.[14] Four mammalian Mo-dependent enzymes are known, all of them harboring a pterin-based molybdenum cofactor (Moco) in their active site: sulfite oxidase, xanthine oxidoreductase, aldehyde oxidase, and mitochondrial amidoxime reductase.[15] People severely deficient in molybdenum have poorly functioning sulfite oxidase and are prone to toxic reactions to sulfites in foods.[16][17] The human body contains about 0.07 mg of molybdenum per kilogram of body weight,[18] with higher concentrations in the liver and kidneys and lower in the vertebrae.[19] Molybdenum is also present within human tooth enamel and may help prevent its decay.[20]
Acute toxicity has not been seen in humans, and the toxicity depends strongly on the chemical state. Studies on rats show a median lethal dose (LD50) as low as 180 mg/kg for some Mo compounds.[21] Although human toxicity data is unavailable, animal studies have shown that chronic ingestion of more than 10 mg/day of molybdenum can cause diarrhea, growth retardation, infertility, low birth weight, and gout; it can also affect the lungs, kidneys, and liver.[22][23] Sodium tungstate is a competitive inhibitor of molybdenum. Dietary tungsten reduces the concentration of molybdenum in tissues.[19]
Low soil concentration of molybdenum in a geographical band from northern China to Iran results in a general dietary molybdenum deficiency, and is associated with increased rates of esophageal cancer.[24][25][26] Compared to the United States, which has a greater supply of molybdenum in the soil, people living in those areas have about 16 times greater risk for esophageal squamous cell carcinoma.[27]
Molybdenum deficiency has also been reported as a consequence of non-molybdenum supplemented total parenteral nutrition (complete intravenous feeding) for long periods of time. It results in high blood levels of sulfite and urate, in much the same way as molybdenum cofactor deficiency. Since pure molybdenum deficiency from this cause occurs primarily in adults, the neurological consequences are not as marked as in cases of congenital cofactor deficiency.[28]
A congenital molybdenum cofactor deficiency disease, seen in infants, is an inability to synthesize molybdenum cofactor, the heterocyclic molecule discussed above that binds molybdenum at the active site in all known human enzymes that use molybdenum. The resulting deficiency results in high levels of sulfite and urate, and neurological damage.[29][30]
Excretion
Most molybdenum is excreted from the human body as molybdate in the urine. Furthermore, urinary excretion of molybdenum increases as dietary molybdenum intake increases. Small amounts of molybdenum are excreted from the body in the feces by way of the bile; small amounts also can be lost in sweat and in hair.[31][32]
Excess and copper antagonism
High levels of molybdenum can interfere with the body's uptake of copper, producing copper deficiency. Molybdenum prevents plasma proteins from binding to copper, and it also increases the amount of copper that is excreted in urine. Ruminants that consume high levels of molybdenum suffer from diarrhea, stunted growth, anemia, and achromotrichia (loss of fur pigment). These symptoms can be alleviated by copper supplements, either dietary or injection.[33] The effective copper deficiency can be aggravated by excess sulfur.[19][34]
Copper reduction or deficiency can also be deliberately induced for therapeutic purposes by the compound ammonium tetrathiomolybdate, in which the bright red anion tetrathiomolybdate is the copper-chelating agent. Tetrathiomolybdate was first used therapeutically in the treatment of copper toxicosis in animals. It was then introduced as a treatment in Wilson's disease, a hereditary copper metabolism disorder in humans; it acts both by competing with copper absorption in the bowel and by increasing excretion. It has also been found to have an inhibitory effect on angiogenesis, potentially by inhibiting the membrane translocation process that is dependent on copper ions.[35] This is a promising avenue for investigation of treatments for cancer, age-related macular degeneration, and other diseases that involve a pathologic proliferation of blood vessels.[36][37]
In some grazing livestock, most strongly in cattle, molybdenum excess in the soil of pasturage can produce scours (diarrhea) if the pH of the soil is neutral to alkaline; see teartness.
References
- ↑ 1.0 1.1 Scott, C.; Lyons, T. W.; Bekker, A.; Shen, Y.; Poulton, S. W.; Chu, X.; Anbar, A. D. (2008). "Tracing the stepwise oxygenation of the Proterozoic ocean". Nature 452 (7186): 456–460. doi:10.1038/nature06811. PMID 18368114. Bibcode: 2008Natur.452..456S.
- ↑ G.J. Leigh. Ch. 5 Structure and Spectroscopic Properties of Metallo-sulfur Clusters Nitrogen Fixation at the Millennium. Elsevier Science B. V., Amsterdam, 2002. 209-210. ISBN:9780444509659.
- ↑ "Biological Nitrogen Fixation" (in en). Annual Review of Biochemistry 14 (1): 685–708. June 1945. doi:10.1146/annurev.bi.14.070145.003345. ISSN 0066-4154.
- ↑ "The nitrogen fixation genes". Nature 239 (5374): 495–9. October 1972. doi:10.1038/239495a0. PMID 4563018. Bibcode: 1972Natur.239..495S.
- ↑ Enemark, John H.; Cooney, J. Jon A.; Wang, Jun-Jieh; Holm, R. H. (2004). "Synthetic Analogues and Reaction Systems Relevant to the Molybdenum and Tungsten Oxotransferases". Chem. Rev. 104 (2): 1175–1200. doi:10.1021/cr020609d. PMID 14871153.
- ↑ Mendel, Ralf R.; Bittner, Florian (2006). "Cell biology of molybdenum". Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1763 (7): 621–635. doi:10.1016/j.bbamcr.2006.03.013. PMID 16784786.
- ↑ Emsley, John (2001). Nature's Building Blocks. Oxford: Oxford University Press. pp. 262–266. ISBN 978-0-19-850341-5. https://books.google.com/books?id=j-Xu07p3cKwC&pg=PA265. Retrieved 2022-07-02.
- ↑ Russ Hille; James Hall; Partha Basu (2014). "The Mononuclear Molybdenum Enzymes". Chem. Rev. 114 (7): 3963–4038. doi:10.1021/cr400443z. PMID 24467397.
- ↑ Kisker, C.; Schindelin, H.; Baas, D.; Rétey, J.; Meckenstock, R. U.; Kroneck, P. M. H. (1999). "A structural comparison of molybdenum cofactor-containing enzymes". FEMS Microbiol. Rev. 22 (5): 503–521. doi:10.1111/j.1574-6976.1998.tb00384.x. PMID 9990727. http://www.ioc.uni-karlsruhe.de/Professoren/Retey/fems_micro_reviews_1999_22_503.pdf. Retrieved 2017-10-25.
- ↑ 10.0 10.1 Mitchell, Phillip C. H. (2003). "Overview of Environment Database". International Molybdenum Association. http://hse.imoa.info/Default.asp?Page=110.
- ↑ Mendel, Ralf R. (2013). "Chapter 15 Metabolism of Molybdenum". in Banci, Lucia. Metallomics and the Cell. Metal Ions in Life Sciences. 12. Springer. doi:10.1007/978-94-007-5561-10_15. ISBN 978-94-007-5560-4. electronic-book ISBN:978-94-007-5561-1 ISSN 1559-0836 electronic-ISSN 1868-0402
- ↑ Chi Chung, Lee; Markus W., Ribbe; Yilin, Hu (2014). "Chapter 7. Cleaving the N,N Triple Bond: The Transformation of Dinitrogen to Ammonia by Nitrogenases". in Peter M.H. Kroneck. The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. 14. Springer. pp. 147–174. doi:10.1007/978-94-017-9269-1_6. ISBN 978-94-017-9268-4.
- ↑ Dos Santos, Patricia C.; Dean, Dennis R. (2008). "A newly discovered role for iron-sulfur clusters". PNAS 105 (33): 11589–11590. doi:10.1073/pnas.0805713105. PMID 18697949. Bibcode: 2008PNAS..10511589D.
- ↑ Schwarz, Guenter; Belaidi, Abdel A. (2013). "Chapter 13. Molybdenum in Human Health and Disease". in Astrid Sigel. Interrelations between Essential Metal Ions and Human Diseases. Metal Ions in Life Sciences. 13. Springer. pp. 415–450. doi:10.1007/978-94-007-7500-8_13. ISBN 978-94-007-7499-5.
- ↑ Mendel, Ralf R. (2009). "Cell biology of molybdenum". BioFactors 35 (5): 429–34. doi:10.1002/biof.55. PMID 19623604.
- ↑ Blaylock Wellness Report, February 2010, page 3.
- ↑ Cohen, H. J.; Drew, R. T.; Johnson, J. L.; Rajagopalan, K. V. (1973). "Molecular Basis of the Biological Function of Molybdenum. The Relationship between Sulfite Oxidase and the Acute Toxicity of Bisulfite and SO2.". Proceedings of the National Academy of Sciences of the United States of America 70 (12 Pt 1–2): 3655–3659. doi:10.1073/pnas.70.12.3655. PMID 4519654. Bibcode: 1973PNAS...70.3655C.
- ↑ Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Academic Press. p. 1384. ISBN 978-0-12-352651-9. https://books.google.com/books?id=vEwj1WZKThEC&pg=PA1384. Retrieved 2022-07-02.
- ↑ 19.0 19.1 19.2 Considine, Glenn D., ed (2005). "Molybdenum". Van Nostrand's Encyclopedia of Chemistry. New York: Wiley-Interscience. pp. 1038–1040. ISBN 978-0-471-61525-5.
- ↑ Curzon, M. E. J.; Kubota, J.; Bibby, B. G. (1971). "Environmental Effects of Molybdenum on Caries". Journal of Dental Research 50 (1): 74–77. doi:10.1177/00220345710500013401.
- ↑ "Risk Assessment Information System: Toxicity Summary for Molybdenum". Oak Ridge National Laboratory. http://rais.ornl.gov/tox/profiles/molybdenum_f_V1.shtml.
- ↑ Coughlan, M. P. (1983). "The role of molybdenum in human biology". Journal of Inherited Metabolic Disease 6 (S1): 70–77. doi:10.1007/BF01811327. PMID 6312191.
- ↑ Barceloux, Donald G.; Barceloux, Donald (1999). "Molybdenum". Clinical Toxicology 37 (2): 231–237. doi:10.1081/CLT-100102422. PMID 10382558.
- ↑ Yang, Chung S. (1980). "Research on Esophageal Cancer in China: a Review". Cancer Research 40 (8 Pt 1): 2633–44. PMID 6992989. http://cancerres.aacrjournals.org/content/40/8_Part_1/2633.full.pdf. Retrieved 2011-12-30.
- ↑ Nouri, Mohsen; Chalian, Hamid; Bahman, Atiyeh; Mollahajian, Hamid et al. (2008). "Nail Molybdenum and Zinc Contents in Populations with Low and Moderate Incidence of Esophageal Cancer". Archives of Iranian Medicine 11 (4): 392–6. PMID 18588371. http://www.ams.ac.ir/AIM/08114/0010.pdf. Retrieved 2009-03-23.
- ↑ Zheng, Liu (1982). "Geographical distribution of trace elements-deficient soils in China". Acta Ped. Sin. 19: 209–223. http://en.cnki.com.cn/Article_en/CJFDTotal-TRXB198203000.htm. Retrieved 2022-07-02.
- ↑ Taylor, Philip R.; Li, Bing; Dawsey, Sanford M.; Li, Jun-Yao; Yang, Chung S.; Guo, Wande; Blot, William J. (1994). "Prevention of Esophageal Cancer: The Nutrition Intervention Trials in Linxian, China". Cancer Research 54 (7 Suppl): 2029s–2031s. PMID 8137333. http://cancerres.aacrjournals.org/content/canres/54/7_Supplement/2029s.full.pdf. Retrieved 2016-07-01.
- ↑ Abumrad, N. N. (1984). "Molybdenum—is it an essential trace metal?". Bulletin of the New York Academy of Medicine 60 (2): 163–71. PMID 6426561.
- ↑ Smolinsky, B; Eichler, S. A.; Buchmeier, S.; Meier, J. C.; Schwarz, G. (2008). "Splice-specific Functions of Gephyrin in Molybdenum Cofactor Biosynthesis". Journal of Biological Chemistry 283 (25): 17370–9. doi:10.1074/jbc.M800985200. PMID 18411266.
- ↑ Reiss, J. (2000). "Genetics of molybdenum cofactor deficiency". Human Genetics 106 (2): 157–63. doi:10.1007/s004390051023. PMID 10746556.
- ↑ Gropper, Sareen S.; Smith, Jack L.; Carr, Timothy P. (2016-10-05) (in en). Advanced Nutrition and Human Metabolism. Cengage Learning. ISBN 978-1-337-51421-7. https://books.google.com/books?id=9-C5DQAAQBAJ. Retrieved 2022-07-02.
- ↑ Turnlund, J. R.; Keyes, W. R.; Peiffer, G. L. (October 1995). "Molybdenum absorption, excretion, and retention studied with stable isotopes in young men at five intakes of dietary molybdenum". The American Journal of Clinical Nutrition 62 (4): 790–796. doi:10.1093/ajcn/62.4.790. ISSN 0002-9165. PMID 7572711.
- ↑ Suttle, N. F. (1974). "Recent studies of the copper-molybdenum antagonism". Proceedings of the Nutrition Society 33 (3): 299–305. doi:10.1079/PNS19740053. PMID 4617883.
- ↑ Hauer, Gerald Copper deficiency in cattle . Bison Producers of Alberta. Accessed Dec. 16, 2010.
- ↑ Nickel, W (2003). "The Mystery of nonclassical protein secretion, a current view on cargo proteins and potential export routes". Eur. J. Biochem. 270 (10): 2109–2119. doi:10.1046/j.1432-1033.2003.03577.x. PMID 12752430.
- ↑ Brewer GJ; Hedera, P.; Kluin, K. J.; Carlson, M.; Askari, F.; Dick, R. B.; Sitterly, J.; Fink, J. K. (2003). "Treatment of Wilson disease with ammonium tetrathiomolybdate: III. Initial therapy in a total of 55 neurologically affected patients and follow-up with zinc therapy". Arch Neurol 60 (3): 379–85. doi:10.1001/archneur.60.3.379. PMID 12633149.
- ↑ Brewer, G. J.; Dick, R. D.; Grover, D. K.; Leclaire, V.; Tseng, M.; Wicha, M.; Pienta, K.; Redman, B. G. et al. (2000). "Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study". Clinical Cancer Research 6 (1): 1–10. PMID 10656425.
 |