Biology:NS5B
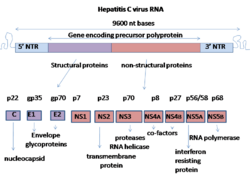
Nonstructural protein 5B (NS5B) is a viral protein found in the hepatitis C virus (HCV).[1] It is an RNA-dependent RNA polymerase, having the key function of replicating HCV's viral RNA by using the viral positive RNA strand as a template to catalyze the polymerization of ribonucleoside triphosphates (rNTP) during RNA replication.[2][3][4] Several crystal structures of NS5B polymerase in several crystalline forms have been determined based on the same consensus sequence BK (HCV-BK, genotype 1).[5] The structure can be represented by a right hand shape with fingers, palm, and thumb. The encircled active site, unique to NS5B, is contained within the palm structure of the protein. Recent studies on NS5B protein genotype 1b strain J4’s (HC-J4) structure indicate a presence of an active site where possible control of nucleotide binding occurs and initiation of de-novo RNA synthesis. De-novo adds necessary primers for initiation of RNA replication.[6]
Drugs targeting NS5B
Several drugs either on the market or in various stages of research target NS5B as a means to prevent further viral RNA replication and thus treat or cure HCV:[7]
- Beclabuvir, currently in clinical trials.
- Dasabuvir (Viekira Pak), non-nucleoside/nucleotide analog, approved by FDA in December 2014 (only in combination with ombitasvir, paritaprevir, and ritonavir).
- Deleobuvir, development terminated.
- Filibuvir, development terminated.
- Radalbuvir, currently in clinical trials.
- Setrobuvir, development terminated.
- Sofosbuvir (Sovaldi; Harvoni (combination with ledipasvir)): nucleotide analog, approved by the FDA in December 2013.
References
- ↑ "Generation of Immune Responses Against HCV Using Dendritic Cells Containing NS5 Protein-Coated Microparticles". Clin. Vaccine Immunol. 16 (2): 163–71. December 2008. doi:10.1128/CVI.00287-08. PMID 19091993.
- ↑ Jin, Z; Leveque, V; Ma, H; Johnson, K. A.; Klumpp, K (2012). "Assembly, purification, and pre-steady-state kinetic analysis of active RNA-dependent RNA polymerase elongation complex". Journal of Biological Chemistry 287 (13): 10674–83. doi:10.1074/jbc.M111.325530. PMID 22303022.
- ↑ Moradpour, D; Penin, F; Rice, CM (2007). "Replication of hepatitis C virus". Nature Reviews. Microbiology 5 (6): 453–63. doi:10.1038/nrmicro1645. PMID 17487147.
- ↑ Rigat, K.; Wang, Y.; Hudyma, T. W.; Ding, M.; Zheng, X.; Gentles, R. G.; Beno, B. R.; Gao, M. et al. (2010). "Ligand-induced changes in hepatitis C virus NS5B polymerase structure". Antiviral Research 88 (2): 197–206. doi:10.1016/j.antiviral.2010.08.014. PMID 20813137.
- ↑ Biswal, B. K.; Cherney, M. M.; Wang, M.; Chan, L.; Yannopoulos, C. G.; Bilimoria, D.; Nicolas, O.; Bedard, J. et al. (2005). "Crystal structures of the RNA-dependent RNA polymerase genotype 2a of hepatitis C virus reveal two conformations and suggest mechanisms of inhibition by non-nucleoside inhibitors". The Journal of Biological Chemistry 280 (18): 18202–18210. doi:10.1074/jbc.M413410200. PMID 15746101.
- ↑ O'Farrell, D; Trowbridge, R; Rowlands, D; Jäger, J (2003). "Substrate complexes of hepatitis C virus RNA polymerase (HC-J4): Structural evidence for nucleotide import and de-novo initiation". Journal of Molecular Biology 326 (4): 1025–35. doi:10.1016/s0022-2836(02)01439-0. PMID 12589751.
- ↑ Biswal, BK; Wang, M; Cherney, MM; Chan, L; Yannopoulos, CG; Bilimoria, D; Bedard, J; James, MN (2006). "Non-nucleoside inhibitors binding to hepatitis C virus NS5B polymerase reveal a novel mechanism of inhibition". Journal of Molecular Biology 361 (1): 33–45. doi:10.1016/j.jmb.2006.05.074. PMID 16828488.
