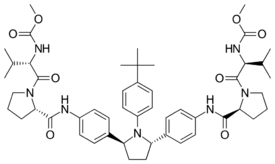
Chemistry:Ombitasvir
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Trade names |
|
| Other names | ABT-267 |
| License data | |
| Routes of administration | By mouth (tablets) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Not determined |
| Protein binding | ~99.9% |
| Metabolism | Amide hydrolysis followed by oxidation |
| Onset of action | ~4 to 5 hours |
| Elimination half-life | 21 to 25 hours |
| Excretion | Mostly with feces (90.2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C50H67N7O8 |
| Molar mass | 894.127 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ombitasvir is an antiviral drug for the treatment of hepatitis C virus (HCV) infection by AbbVie. In the United States, it is approved by the Food and Drug Administration for use in combination with paritaprevir, ritonavir and dasabuvir in the product Viekira Pak for the treatment of HCV genotype 1,[1][2] and with paritaprevir and ritonavir in the product Technivie for the treatment of HCV genotype 4.[3][4]
Ombitasvir is an NS5A inhibitor that acts by inhibiting the HCV protein NS5A.[5]
See also
- Discovery and development of NS5A inhibitors
References
- ↑ "VIEKIRA PAK™ (ombitasvir, paritaprevir and ritonavir tablets; dasabuvir tablets), for Oral Use. Full Prescribing Information". AbbVie Inc., North Chicago, IL 60064. http://www.rxabbvie.com/pdf/viekirapak_pi.pdf.
- ↑ "FDA approves Viekira Pak to treat hepatitis C". Food and Drug Administration. December 19, 2014. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427530.htm.
- ↑ "TECHNIVIE™ (ombitasvir, paritaprevir and ritonavir) Tablets, for Oral Use. Full Prescribing Information". AbbVie Inc., North Chicago, IL 60064. http://rxabbvie.com/pdf/technivie_pi.pdf.
- ↑ "FDA approves Technivie for treatment of chronic hepatitis C genotype 4". Food and Drug Administration. July 24, 2015. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455857.htm.
- ↑ "Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin". The New England Journal of Medicine 370 (17): 1594–603. April 2014. doi:10.1056/NEJMoa1315722. PMID 24720703. https://espace.library.uq.edu.au/view/UQ:330486/UQ330486_OA.pdf.
Further reading
- "Efficacy and Safety of Ombitasvir/Paritaprevir/Ritonavir and Dasabuvir With or Without Ribavirin in Patients With Chronic Hepatitis C Virus Genotype 1 Infection Receiving Opioid Substitution Therapy: A Post Hoc Analysis of 12 Clinical Trials". Open Forum Infectious Diseases 5 (11): ofy248. November 2018. doi:10.1007/s15010-018-1157-x. OCLC 1105037362. PMID 30430131.
- "Ombitasvir, paritaprevir, and ritonavir, with or without dasabuvir, plus ribavirin for patients with hepatitis C virus genotype 1 or 4 infection with cirrhosis (ABACUS): a prospective observational study". The Lancet. Gastroenterology & Hepatology 2 (6): 427–434. June 2017. doi:10.1016/S2468-1253(17)30048-1. PMID 28497758.
 |

