Chemistry:Ledipasvir
 | |
| Clinical data | |
|---|---|
| Trade names | Harvoni (combination with sofosbuvir) |
| Other names | GS-5885 |
| License data | |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 76% |
| Protein binding | >99% |
| Metabolism | No cytochrome metabolism |
| Elimination half-life | 47 hrs |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
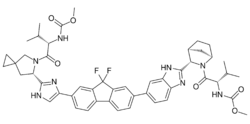
| Formula | C49H54F2N8O6 |
| Molar mass | 889.018 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences.[1] After completing Phase III clinical trials, on February 10, 2014, Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C.[2][3] The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin.
Ledipasvir is an inhibitor of NS5A, a hepatitis C virus protein.[citation needed]
Data presented at the 20th Conference on Retroviruses and Opportunistic Infections in March 2013 showed that a triple regimen of the nucleotide analog inhibitor sofosbuvir, ledipasvir, and ribavirin produced a 12-week post-treatment sustained virological response (SVR12) rate of 100% for both treatment-naive patients and prior non-responders with HCV genotype 1.[4][5] The sofosbuvir/ledipasvir coformulation is being tested with and without ribavirin. In February 2014 Gilead filed for United States Food and Drug Administration (FDA) approval of ledipasvir/sofosbuvir oral treatment, without interferon and ribavirin.[6]
On 10 October 2014 the FDA approved the combination product ledipasvir/sofosbuvir called Harvoni.[7]
Medical uses
Ledipasvir is most commonly used in combination with sofosbuvir for treatment in chronic hepatitis C genotype 1 patients. This drug has been tested and shown efficacy in treatment-naive and treatment experienced patients.[8]
Adverse effects
According to clinical trials, ledipasvir/sofosbuvir has been very well tolerated with the most common side effects being fatigue and headache.[9]
Interactions
Most drug-drug interactions with Harvoni involve Pgp-inducers such as St. John’s wort or rifampicin. Concomitant use will decrease the blood concentration of Harvoni and thus, have reduced therapeutic effects.[9]
Mechanism of action
Ledipasvir inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion.[10]
Sofosbuvir, on the other hand, is metabolized to a uridine triphosphate mimic, which acts as a RNA chain terminator when incorporated into RNA by NS5B polymerase.[10]
Cost
Similar to sofosbuvir, the cost of Harvoni has been a controversial topic. It costs $1,125 per pill in the US, translating to $63,000 for an 8-week treatment course, $94,500 for a 12-week treatment course, or $189,000 for a 24-week treatment course. Gilead justifies the cost by outweighing the benefit of curing hepatitis C over the cost of spending double on liver transplants or temporarily treating liver diseases. Gilead has provided a ledipasvir/sofosbuvir assistance program for eligible underserved or underinsured hepatitis C patients who cannot afford the costs of treatment.[10]
In July 2015 Gilead modified the eligibility criteria to receive Support Path benefits for HCV patients in the United States.[citation needed]
See also
- Discovery and development of NS5A inhibitors
References
- ↑ "Ledipasvir". United States Adopted Name. http://www.ama-assn.org/resources/doc/usan/ledipasvir.pdf.
- ↑ "Ledipasvir-submitted-to-FDA". http://www.gilead.com/news/press-releases/2014/2/gilead-files-for-us-approval-of-ledipasvirsofosbuvir-fixeddose-combination-tablet-for-genotype-1-hepatitis-c#sthash.nk1apkTf.dpuf..
- ↑ "GS-5885". Gilead Sciences. http://www.gilead.com/pipeline.
- ↑ ELECTRON: 100% Suppression of Viral Load through 4 Weeks’ Post-treatment for Sofosbuvir + Ledipasvir (GS-5885) + Ribavirin for 12 Weeks in Treatment-naïve and -experienced Hepatitis C Virus GT 1 Patients . Gane, Edward et al. 20th Conference on Retroviruses and Opportunistic Infections. March 3–6, 2013. Abstract 41LB.
- ↑ CROI 2013: Sofosbuvir + Ledipasvir + Ribavirin Combo for HCV Produces 100% Sustained Response . Highleyman, Liz. HIVandHepatitis.com. 4 March 2013.
- ↑ "Gilead Files for U.S. Approval of Ledipasvir/Sofosbuvir Fixed-Dose Combination Tablet for Genotype 1 Hepatitis C". Gilead Sciences. 10 February 2014. http://www.gilead.com/news/press-releases/2014/2/gilead-files-for-us-approval-of-ledipasvirsofosbuvir-fixeddose-combination-tablet-for-genotype-1-hepatitis-c.
- ↑ "U.S. Food and Drug Administration Approves Gilead's Harvoni (Ledipasvir/Sofosbuvir), the First Once-Daily Single Tablet Regimen for the Treatment of Genotype 1 Chronic Hepatitis C". 10 October 2014. http://www.gilead.com/news/press-releases/2014/10/us-food-and-drug-administration-approves-gileads-harvoni-ledipasvirsofosbuvir-the-first-oncedaily-single-tablet-regimen-for-the-treatment-of-genotype-1-chronic-hepatitis-c.
- ↑ "Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection". The New England Journal of Medicine 370 (20): 1889–98. May 2014. doi:10.1056/NEJMoa1402454. PMID 24725239.
- ↑ 9.0 9.1 "PRESCRIBING INFORMATION". https://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf.
- ↑ 10.0 10.1 10.2 "Ledipasvir-Sofosbuvir Harvoni - Treatment - Hepatitis C Online". https://www.hepatitisc.uw.edu/page/treatment/drugs/ledipasvir-sofosbuvir.
 |
