Biology:Prokaryotic riboflavin biosynthesis protein
The prokaryotic riboflavin biosynthesis protein is a bifunctional enzyme found in bacteria that catalyzes the phosphorylation of riboflavin into flavin mononucleotide (FMN) and the adenylylation of FMN into flavin adenine dinucleotide (FAD). It consists of a C-terminal riboflavin kinase and an N-terminal FMN-adenylyltransferase. This bacterial protein is functionally similar to the monofunctional riboflavin kinases and FMN-adenylyltransferases of eukaryotic organisms, but only the riboflavin kinases are structurally homologous.
Structure
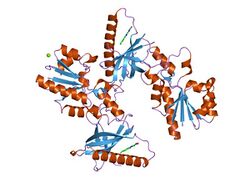
Prokaryotic riboflavin biosynthesis proteins are also known as the prokaryotic type-I FAD synthetases, which consist of a C-terminal riboflavin kinase (RFK) and an N-terminal FMN-adenylyltransferase (FMNAT). The globular RFK consists of six antiparallel β-sheets that form a β-barrel, and an α-helix adjacent to this structure. The barrel and helix are held together by 7 independent loops.[1] The FMNAT module contains an α/β dinucleotide binding domain within the active site, which it uses to bind to the substrate. The overall structure is held together by 5 parallel β-sheets that are adjacent to 4 α-helices, with 2 being long and 2 being short. A subdomain, containing 2 smaller α-helices, encompasses the area that connects to the C-terminal RFK module.[2]
Mechanism
Riboflavin is converted into catalytically active cofactors FAD and FMN by the actions of riboflavin kinase EC 2.7.1.26, which converts it into FMN, and FAD synthetase EC 2.7.7.2, which adenylates FMN to FAD. The RFK module phosphorylates the riboflavin substrate and converts it into FMN, which is then released from the module. This reaction is dependent on an ATP molecule stabilized by an Mg2+ ion, which causes only a single phosphate group to leave the ATP and bond to riboflavin. The released FMN then joins to the N-terminal FMNAT module and is adenylated, with the adenylyl group of ATP attaching to the phosphate group on FMN and the diphosphate group leaving.[1]
ATP + riboflavin ⇌ ADP + FMN
ATP + FMN ⇌ diphosphate + FAD
Phylogenetic Domain Comparison
Eukaryotes usually have two separate enzymes, while most prokaryotes have a single bifunctional protein that can carry out both catalyses, although exceptions occur in both cases. While eukaryotic monofunctional RFK is orthologous to the bifunctional prokaryotic RFK module, the monofunctional FMNAT differs from its prokaryotic counterpart, and is instead related to the PAPS-reductase family.[3][4] The bacterial FMNAT module of the bifunctional enzyme has remote similarity to eukaryotic nucleotidyltransferases and, hence, it may be involved in the adenylylation reaction of FAD synthetases.[5]
References
- ↑ Jump up to: 1.0 1.1 "Kinetics and thermodynamics of the protein-ligand interactions in the riboflavin kinase activity of the FAD synthetase from Corynebacterium ammoniagenes". Scientific Reports 7 (1): 7281. August 2017. doi:10.1038/s41598-017-07875-5. PMID 28779158. Bibcode: 2017NatSR...7.7281S.
- ↑ "Structural analysis of FAD synthetase from Corynebacterium ammoniagenes". BMC Microbiology 8 (1): 160. September 2008. doi:10.1186/1471-2180-8-160. PMID 18811972.
- ↑ "Ligand binding-induced conformational changes in riboflavin kinase: structural basis for the ordered mechanism". Biochemistry 42 (43): 12532–8. November 2003. doi:10.1021/bi035450t. PMID 14580199.
- ↑ "Over-expression in Escherichia coli, purification and characterization of isoform 2 of human FAD synthetase". Protein Expression and Purification 52 (1): 175–81. March 2007. doi:10.1016/j.pep.2006.09.002. PMID 17049878.
- ↑ "A conserved domain in prokaryotic bifunctional FAD synthetases can potentially catalyze nucleotide transfer". Trends in Biochemical Sciences 28 (1): 9–12. January 2003. doi:10.1016/S0968-0004(02)00009-9. PMID 12517446.
 |