Biology:Protocadherin
| Protocadherin, cytoplasmic | |||||||||
|---|---|---|---|---|---|---|---|---|---|
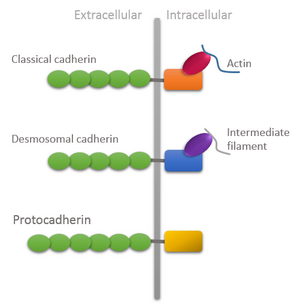 Domain organization of different types of cadherins showing unique features of protocadherins: Extracellular domain is longer and intracellular domain lacks attachment with cytoskeleton. | |||||||||
| Identifiers | |||||||||
| Symbol | PDCH | ||||||||
| Pfam | PF08374 | ||||||||
| InterPro | IPR013585 | ||||||||
| Membranome | 114 | ||||||||
| |||||||||
Protocadherins (Pcdhs) are the largest mammalian subgroup of the cadherin superfamily of homophilic cell-adhesion proteins.[1] They were discovered by Shintaro Suzuki's group, when they used PCR to find new members of the cadherin family. The PCR fragments that corresponded to protocadherins were found in vertebrate and invertebrate species.[2] This prevalence in a wide range of species suggested that the fragments were part of an ancient cadherin and were thus termed "Protocadherins" as the "first cadherins". Of the approximately 70 Pcdh genes identified in mammalian genomes, over 50 are located in tightly linked gene clusters on the same chromosome.[3] Until recently, it was assumed that this kind of organization can only be found in vertebrates,[3] but Octopus bimaculoides has 168 genes of which nearly three-quarters are found in tandem clusters with the two largest clusters compromising 31 and 17 genes, respectively.[4]
Classification
In mammals, two types of Pcdh genes have been defined: the non-clustered Pcdhs which are scattered throughout the genome; and the clustered Pcdhs organized in three gene clusters designated α, β, γ which in mouse genome comprises 14, 22 and 22, respectively, large variable exons arrayed in tandem. Each exon is transcribed from its owner promoter and encodes: the entire extracellular domain, a transmembrane domain, and a short and variable intracellular domain of the corresponding Pcdh protein which differs from the Cadherin intracellular domain due to lack of attachment to the cytoskeleton through catenins.[5]
Moreover, these clustered Pcdh genes are predominantly expressed in the developing nervous system[2] and since different subsets of Pcdhs genes are differentially expressed in individual neurons, a vast cell surface diversity may arise from this combinatorial expression.[5] This has led to speculation and further to the proposal that Pcdhs may provide a synaptic-address code for neuronal connectivity or a single-cell barcode for self-recognition/self-avoidance similar to that ascribed to DSCAM proteins of invertebrates. Although vertebrate DSCAMs lack the diversity of their invertebrate counterparts, the selective transcription of individual Pcdh isoforms can be achieved by promoter choice followed by alternative pre-mRNA cis-splicing thus increasing the number of possible combinations.
Function
Homophilic interactions and intracellular signaling
Clustered Pcdhs proteins are detected throughout the neuronal soma, dendrites and axons and are observed in synapses and growth cones.[6][7][8][9][10] Like classical cadherins, members of Pcdhs family were also shown to mediate cell-cell adhesion in cell-based assays[11][12][13] and most of them showed to engage in homophilic trans-interactions.[14] Schreiner and Weiner [14] showed that Pcdhα and γ proteins can form multimeric complexes. If all three classes of Pcdhs could engage in multimerization of stochastically expressed Pcdhs isoforms, then neurons could produce a large number of distinct homophilic interaction units, amplifying significantly the cell-surface diversity more than the one afforded by stochastic gene expression alone.
As for cytoplasmic domain, all the three classes of clustered Pcdhs proteins are dissimilar, although they are strictly conserved in vertebrate evolution, suggesting a conserved cellular function.[5] This is corroborated by a large number of other interacting proteins including phosphatases, kinases, adhesion molecules and synaptic proteins[15] The cytoplasmic domain also mediates intracellular retention, a property which distinguishes the clustered protocadherins from the related classical cadherins.[16] Furthermore, it was shown that Pcdhs are proteolytically processed by γ-secretase complex,[17][18] which releases soluble intracellular fragments into the cytoplasm which might have a broad range of functions as acting locally in the cytoplasm and/or even regulate gene expression similarly to other cell-surface proteins such as Notch and N-cadherin. Since these molecules are involved in so many developmental processes like axon guidance and dendrite arborization, mutations in Pcdhs genes and their expression may play a role in Down, Rett as well as Fragile X syndrome,[19] schizophrenia,[20] and neurodegenerative diseases[21]
The cytoplasmic domain of Pcdh-alpha can be divided into two specific types. Both of them enhance homophilic interactions. They associate with neurofillament M and fascin respectively.[22]
Human genes
- PCDH1
- PCDH7
- PCDH8
- PCDH9
- PCDH10
- PCDH11X/11Y
- PCDH12
- PCDH15
- PCDH17
- PCDH18
- PCDH19
- PCDH20
- PCDHA1
- PCDHA2
- PCDHA3
- PCDHA4
- PCDHA5
- PCDHA6
- PCDHA7
- PCDHA8
- PCDHA9
- PCDHA10
- PCDHA11
- PCDHA12
- PCDHA13
- PCDHAC1
- PCDHAC2
- PCDHB1
- PCDHB2
- PCDHB3
- PCDHB4
- PCDHB5
- PCDHB6
- PCDHB7
- PCDHB8
- PCDHB9
- PCDHB10
- PCDHB11
- PCDHB12
- PCDHB13
- PCDHB14
- PCDHB15
- PCDHB16
- PCDHB17
- PCDHB18
- PCDHGA1
- PCDHGA2
- PCDHGA3
- PCDHGA4
- PCDHGA5
- PCDHGA6
- PCDHGA7
- PCDHGA8
- PCDHGA9
- PCDHGA10
- PCDHGA11
- PCDHGA12
- PCDHGB1
- PCDHGB2
- PCDHGB3
- PCDHGB4
- PCDHGB5
- PCDHGB6
- PCDHGB7
- PCDHGC3
- PCDHGC4
- PCDHGC5
- FAT
- FAT2
- FAT4
See also
- Cadherin
- PCDH11X
- Neuronal self-avoidance
- Epileptic Encephalopathy, Early Infantile, 9, caused by mutation in the gene encoding protocadherin-19
References
- ↑ "Molecular evolution of the cadherin superfamily". The International Journal of Biochemistry & Cell Biology 41 (2): 349–69. February 2009. doi:10.1016/j.biocel.2008.09.027. PMID 18848899.
- ↑ 2.0 2.1 "Protocadherins: a large family of cadherin-related molecules in central nervous system". The EMBO Journal 12 (6): 2249–56. June 1993. doi:10.1002/j.1460-2075.1993.tb05878.x. PMID 8508762.
- ↑ 3.0 3.1 "Functional significance of isoform diversification in the protocadherin gamma gene cluster". Neuron 75 (3): 402–9. August 2012. doi:10.1016/j.neuron.2012.06.039. PMID 22884324.
- ↑ Albertin, Caroline B.; Simakov, Oleg; Mitros, Therese; Wang, Z. Yan; Pungor, Judit R.; Edsinger-Gonzales, Eric; Brenner, Sydney; Ragsdale, Clifton W. et al. (August 2015). "The octopus genome and the evolution of cephalopod neural and morphological novelties". Nature 524 (7564): 220–224. doi:10.1038/nature14668. ISSN 0028-0836. PMID 26268193. Bibcode: 2015Natur.524..220A.
- ↑ 5.0 5.1 5.2 "Clustered protocadherins". Development 140 (16): 3297–302. August 2013. doi:10.1242/dev.090621. PMID 23900538.
- ↑ "Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex". Neuron 20 (6): 1137–51. June 1998. doi:10.1016/s0896-6273(00)80495-x. PMID 9655502.
- ↑ "Gamma protocadherins are required for survival of spinal interneurons". Neuron 36 (5): 843–54. December 2002. doi:10.1016/s0896-6273(02)01090-5. PMID 12467588.
- ↑ "Changes in subcellular distribution of protocadherin gamma proteins accompany maturation of spinal neurons". Journal of Neuroscience Research 72 (5): 549–56. June 2003. doi:10.1002/jnr.10618. PMID 12749019.
- ↑ "Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons". The Journal of Neuroscience 23 (12): 5096–104. June 2003. doi:10.1523/JNEUROSCI.23-12-05096.2003. PMID 12832533.
- ↑ "Postsynaptic and differential localization to neuronal subtypes of protocadherin beta16 in the mammalian central nervous system". The European Journal of Neuroscience 27 (3): 559–71. February 2008. doi:10.1111/j.1460-9568.2008.06052.x. PMID 18279309.
- ↑ "Protocadherin Pcdh2 shows properties similar to, but distinct from, those of classical cadherins". Journal of Cell Science 108 ( Pt 12) (12): 3765–73. December 1995. doi:10.1242/jcs.108.12.3765. PMID 8719883.
- ↑ "Differential expression of individual gamma-protocadherins during mouse brain development". Molecular and Cellular Neurosciences 29 (4): 603–16. August 2005. doi:10.1016/j.mcn.2005.05.001. PMID 15964765.
- ↑ "Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion". The Journal of Biological Chemistry 281 (31): 21735–44. August 2006. doi:10.1074/jbc.M602663200. PMID 16751190.
- ↑ 14.0 14.1 "Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion". Proceedings of the National Academy of Sciences of the United States of America 107 (33): 14893–8. August 2010. doi:10.1073/pnas.1004526107. PMID 20679223. Bibcode: 2010PNAS..10714893S.
- ↑ "Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret". Proceedings of the National Academy of Sciences of the United States of America 107 (31): 13894–9. August 2010. doi:10.1073/pnas.1007182107. PMID 20616001.
- ↑ "Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons". Molecular and Cellular Neurosciences 40 (3): 344–53. March 2009. doi:10.1016/j.mcn.2008.12.002. PMID 19136062.
- ↑ "Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing". Molecular and Cellular Biology 27 (11): 4121–32. June 2007. doi:10.1128/MCB.01708-06. PMID 17403907.
- ↑ "Proteolytic processing of protocadherin proteins requires endocytosis". Proceedings of the National Academy of Sciences of the United States of America 107 (41): 17774–9. October 2010. doi:10.1073/pnas.1013105107. PMID 20876099. Bibcode: 2010PNAS..10717774B.
- ↑ "Dendritic anomalies in disorders associated with mental retardation". Cerebral Cortex 10 (10): 981–91. October 2000. doi:10.1093/cercor/10.10.981. PMID 11007549.
- ↑ "Evidence for positive selection on Protocadherin Y gene in Homo sapiens: implications for schizophrenia". Schizophrenia Research 108 (1–3): 299–300. March 2009. doi:10.1016/j.schres.2008.09.015. PMID 18938061.
- ↑ "Dendritic changes in Alzheimer's disease and factors that may underlie these changes". Progress in Neurobiology 55 (6): 595–609. August 1998. doi:10.1016/s0301-0082(98)00022-7. PMID 9670220.
- ↑ "Cytoplasmic domain of protocadherin-alpha enhances homophilic interactions and recognizes cytoskeletal elements.". Journal of Neurobiology 66 (4): 393–407. March 2006. doi:10.1002/neu.20228. PMID 16408303.
Further reading
- "Proteomics analysis reveals overlapping functions of clustered protocadherins". Molecular & Cellular Proteomics 9 (1): 71–83. January 2010. doi:10.1074/mcp.M900343-MCP200. PMID 19843561.
- "Sorting out a promiscuous superfamily: towards cadherin connectomics". Trends in Cell Biology 24 (9): 524–36. September 2014. doi:10.1016/j.tcb.2014.03.007. PMID 24794279.
- "PProtocadherins at the Crossroad of Signaling Pathways". Frontiers in Molecular Neuroscience 13: 117. June 2020. doi:10.3389/fnmol.2020.00117. PMID 32694982.
 |
