Biology:Septate junction
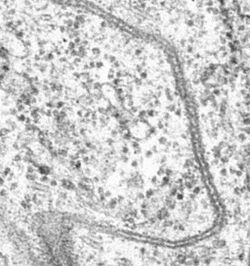
Septate junctions are intercellular junctions found in invertebrate epithelial cells, appearing as ladder-like structures under electron microscopy. They are thought to provide structural strength and a barrier to solute diffusion through the intercellular space. They are considered somewhat analogous to the (vertebrate) tight junctions; however, tight and septate junctions are different in many ways. Known insect homologues of tight junction components are components of conserved signalling pathways that localize to either adherens junctions, the subapical complex, or the marginal zone.[1] Recent studies show that septate junctions are also identified in the myelinated nerve fibers of the vertebrates.[2][3]
Structure
The main trait of septate junctions structure is that cross-bridges or septa are in the ladder-like shape and cover the 15–20 nm intermembrane space of cell–cell contacts. Septate junctions are in a tight arrangement which is parallel to each other.[4]
For the septate junctions, several components are related to the function or the morphology of septate junctions, like Band 4.1-Coracle, Discs-large, fasciclin III, Neurexin IV (NRX) and so on.[5][6] Band 4.1-Coracle is necessary for the interaction of the cell.[6] Discs-large, a key component of septate junctions, is needed for the growth control.[7][8] Fasciclin III acts as an adhesion protein.[6] Neurexin IV (NRX) is a required transmembrane protein for the formation of septate junctions. For example, the glial–glial septate junctions that lack NRX will cause the blood barriers to break down.[5] Gliotactin (Gli), is also a necessary a transmembrane protein for the formation of pleated septate junctions.[4][9] Tsp2A and Undicht are newly identified components that are needed for the formation of smooth septate junctions and septate junctions.[10][11]
There are three known claudins contained in the septate junctions, Megatrachea (Mega), Sinuous (Sinu) and Kune-kune (Kune). Among these three claudins, Kune-kune (Kune) plays a more central role in septate junctions organization and function.[12]
Function
There are several functions of septate junctions.
- Pleated SJs(pSJs) play roles in development and cell signaling.[5]
- Form the mechanical link between cells which can densely pack the epithelial sheaths.[5]
- Intermediate the adjacent cells interaction.[6]
- Prevent the free diffusion of water and solutes among adjacent epithelial cells.[2][13]
- Preserve the epithelial polarity and cell adhesion.[2]
- Have a function in the morphogenesis like tracheal morphology that regulate the cell size and the cell length.[14]
- Regulate cell proliferation.[14]
- Characterize the properties of the paracellular pathway in insect Malpighian tubules (MTs).[9]
- Some unusual septate junctions have the function in the Drosophila testis like keeping premature sperm staying inside.[15]
For the septate junctions in the vertebrates, they play some roles of tight junctions.[14]
Na+/K+ ATPase works for the function of septate junctions.[16]
Classification
In Drosophila melanogaster, there are two types of septate junctions, smooth SJs (sSJs) and pleated SJs(pSJs). sSJs and pSJs are distributed in different tissues. sSJs are in gut endoderm and Malpighian tubules, while pSJs are in the ectodermally derived epithelia.[5] sSJs and pSJs vary in shape but have the same function.[4]
See also
References
- ↑ "Signalling to and from tight junctions". Nature Reviews. Molecular Cell Biology 4 (3): 225–36. March 2003. doi:10.1038/nrm1055. PMID 12612641.
- ↑ 2.0 2.1 2.2 "Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function". Development 131 (20): 4931–42. October 2004. doi:10.1242/dev.01372. PMID 15459097.
- ↑ "Neuroglian, Gliotactin, and the Na+/K+ ATPase are essential for septate junction function in Drosophila". The Journal of Cell Biology 161 (5): 979–89. June 2003. doi:10.1083/jcb.200212054. PMID 12782686.
- ↑ 4.0 4.1 4.2 "Gliotactin, a novel marker of tricellular junctions, is necessary for septate junction development in Drosophila". The Journal of Cell Biology 161 (5): 991–1000. June 2003. doi:10.1083/jcb.200303192. PMID 12782681.
- ↑ 5.0 5.1 5.2 5.3 5.4 "A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function". Cell 87 (6): 1059–68. December 1996. doi:10.1016/s0092-8674(00)81800-0. PMID 8978610.
- ↑ 6.0 6.1 6.2 6.3 "Drosophila coracle, a member of the protein 4.1 superfamily, has essential structural functions in the septate junctions and developmental functions in embryonic and adult epithelial cells". Molecular Biology of the Cell 9 (12): 3505–19. December 1998. doi:10.1091/mbc.9.12.3505. PMID 9843584.
- ↑ "The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions". Cell 66 (3): 451–64. August 1991. doi:10.1016/0092-8674(81)90009-x. PMID 1651169.
- ↑ Ward, Robert E.; Lamb, Rebecca S.; Fehon, Richard G. (1998-03-23). "A Conserved Functional Domain ofDrosophilaCoracle Is Required for Localization at the Septate Junction and Has Membrane-organizing Activity". The Journal of Cell Biology 140 (6): 1463–1473. doi:10.1083/jcb.140.6.1463. ISSN 0021-9525. PMID 9508778.
- ↑ 9.0 9.1 Kolosov, Dennis; Jonusaite, Sima; Donini, Andrew; Kelly, Scott P.; O'Donnell, Michael J. (2019-05-07). "Septate junction in the distal ileac plexus of larval lepidopteran Trichoplusia ni: alterations in paracellular permeability during ion transport reversal". The Journal of Experimental Biology 222 (11): jeb204750. doi:10.1242/jeb.204750. ISSN 0022-0949. PMID 31064858.
- ↑ Izumi, Yasushi; Motoishi, Minako; Furuse, Kyoko; Furuse, Mikio (2016-04-01). "A tetraspanin regulates septate junction formation inDrosophilamidgut". Development 143 (7): e1.1. doi:10.1242/dev.137646. ISSN 0950-1991. http://jcs.biologists.org/cgi/content/short/129/6/1155.
- ↑ Petri, Johanna; Syed, Mubarak Hussain; Rey, Simone; Klämbt, Christian (February 2019). "Non-Cell-Autonomous Function of the GPI-Anchored Protein Undicht during Septate Junction Assembly". Cell Reports 26 (6): 1641–1653.e4. doi:10.1016/j.celrep.2019.01.046. ISSN 2211-1247. PMID 30726744.
- ↑ Nelson, Kevin S.; Furuse, Mikio; Beitel, Greg J. (2010-04-20). "The Drosophila Claudin Kune-kune Is Required for Septate Junction Organization and Tracheal Tube Size Control". Genetics 185 (3): 831–839. doi:10.1534/genetics.110.114959. ISSN 0016-6731. PMID 20407131.
- ↑ Wu VM. Sinuous and varicose function to organize the septate junction complex and regulate tracheal tube size control (Ph. D. thesis). Northwestern University. ISBN 978-0-542-74446-4. OCLC 157010952.
- ↑ 14.0 14.1 14.2 Llimargas, M. (2004-01-01). "Lachesin is a component of a septate junction-based mechanism that controls tube size and epithelial integrity in the Drosophila tracheal system". Development 131 (1): 181–190. doi:10.1242/dev.00917. ISSN 0950-1991. PMID 14681183.
- ↑ Dubey, Pankaj; Kapoor, Tushna; Gupta, Samir; Shirolikar, Seema; Ray, Krishanu (2018-07-01). Atypical septate junctions maintain the somatic enclosure around maturing spermatids and prevent premature sperm release in Drosophila testis. doi:10.1101/360115.
- ↑ "The Na+/K+ ATPase is required for septate junction function and epithelial tube-size control in the Drosophila tracheal system". Development 130 (20): 4963–74. October 2003. doi:10.1242/dev.00691. PMID 12930776.
 |
