Biology:Shavenbaby
| Transcriptional regulator ovo | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Organism | |||||||
| Symbol | ovo | ||||||
| UniProt | P51521 | ||||||
| |||||||
Overview of shavenbaby (svb)
The shavenbaby (svb) or ovo gene encodes a transcription factor in Drosophila responsible for inducing cells to become hair-like projections called trichomes or microtrichia.[1][2] Many of the major developmental signaling pathways converge at the shavenbaby locus, which then regulates over 150 downstream target genes.[3] The "hourglass" shape of this gene regulatory network makes shavenbaby the master regulator of trichome formation.[4][2] The unique setup of the gene regulatory network made trichomes an excellent readout to identify important developmental genes during the forward genetics Heidelberg Screen.[5] Additionally, shavenbaby is considered to be an "evolutionary hotspot",[6] and experiments have shown that changes in this gene cause the loss of dorsal cuticular hairs in Drosophila sechellia larvae.[2]
Trichomes likely serve a variety of purposes. In larvae, trichomes likely help with larval locomotion. By alternating between bands of trichomes and naked cuticle, larvae can tread across different surfaces. Additionally, trichomes may contribute to hydrophobicity and even stabilize adult flight.[7]
Transcriptional inputs for svb
The shavenbaby locus is regulated by multiple signaling pathways, including the HOX factors, Wingless, EGF-R, Hedgehog, and Notch signaling.[1][8] Additionally, the transcription factors SoxNeuro, Pointed, and Dichaete regulate shavenbaby expression.[9]
Engrailed and Hedgehog activate EGFR
During stage 12 of embryonic development, Engrailed is expressed in a subset of cells, which activates the hedgehog signaling pathway. The Hedgehog signal is received by cells expressing Patched, which induces expression of rhomboid (rho) with Serrate-Notch signaling, which activates the EGFR signaling pathway. The drosophila EGF receptor (DER) is responsible for activating shavenbaby both directly and by driving expression of the factors SoxNeuro and Dichaete.[8][9][1][10] Other transcription factors such as Ultrabithorax and its cofactor Homothorax also interact with the different shavenbaby enhancers to activate expression.[11][12]
Wingless signaling represses shavenbaby
During stage 12, the Hedgehog signaling pathway induces expression of the Wingless signal. The Wingless signaling pathway is responsible for repressing shavenbaby activity, and cells expressing Wingless have naked cuticle. Furthermore, mutations to the Wingless gene produce a lawn of trichomes in the naked region. Wingless signaling has been characterized to specifically integrate at the shavenbaby E3 enhancer, which also produces a lawn of expression in Wingless mutants.[10][12] Wingless signaling is repressed by both SoxNeuro and Dichaete, products of the EGFR signaling pathway.[9]
Developmental enhancers of svb
Developmental enhancers are DNA sequences which control the spatial-temporal patterning of genes during development to set up the bodyplan of an organism.[13] Developmental enhancers are thought to be the main drivers of phenotypic evolution.[6][9] There are currently seven putative developmental enhancers in the shavenbaby locus: DG2, DG3, Z1.3, A, E3, E6, and 7H.[14][15] All of these enhancers are pleiotropic, expressing shavenbaby across different developmental stages.[15] The enhancers are somewhat modular, where different patterning components are partitioned to different enhancers. However, many of the expression patterns overlap with each other making the enhancers seemingly redundant.[7]
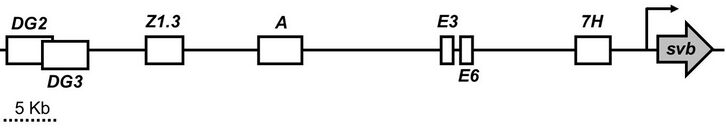
Enhancer redundancy is a commonly observed phenomenon.[16] Why would evolution evolve redundant enhancers? The mystery of enhancer redundancy was partially resolved by studying the shavenbaby locus in 2010.[17] Frankel et al. found that the redundant enhancers help maintain proper shavenbaby expression under different temperature stresses,[17] canalizing its expression. This finding was also observed eight years later for redundant mammalian enhancers,[18] suggesting that this observation is not limited to Drosophila. Redundant enhancers have also been observed to use different transcription factors, incorporating a diverse set of signaling inputs to canalize gene expression under different environmental stresses.[19]
The E3 enhancer
The E3 enhancer is a 1,042 base-pair (bp) enhancer which drives shavenbaby on the ventral side of stages 15 and 16+ embryos and larvae. E3 is also expressed pleiotropically in the pharynx and esophagus or third-instar larvae. In adult Drosophila, E3 is expressed in the abdomen, head, legs, and wing.[7] The E3 fragment has been tested as smaller fragments such as E3-14[20] and E3N.[12][11] Unlike the other shavenbaby enhancers, E3 activity is maintained in Drosophila sechellia.[15][12]
E3N was first described in Crocker et al., 2015,[11] and was found to encode "homotypic clusters" of binding sites for the transcription factor: Ultrabithorax (Ubx). These binding sites, however, were non-canonical, and Ubx binds to E3N at a very low-affinity.[11] Mutations to increase the affinity of these binding sites caused the ectopic binding of other Homeobox (HOX) factors, resulting in ectopic enhancer expression. HOX factors license the identity of cells, locking them into a fate to produce a particular structure such as wings, halteres, antennae, abdomen, etc.[21] All of the HOX factors are evolutionarily related, and bind to the same homeodomain sequence: TAAT. How enhancers encode the specific binding of certain HOX factors and prevent the ectopic binding of others is called the "Hox Paradox". The E3N study from Crocker et al., 2015 provided an answer to the "Hox Paradox",[22] by suggesting that low-affinity binding sites would provide the specificity, and encoding clusters of the sites would account for the potential weak activation.[11] Low-affinity transcription factor binding sites have also been observed in other enhancers.[23]
In a follow-up study, Fuqua et al. created a library of random mutants to the E3N enhancer to study the enhancer grammar and how enhancers can evolve.[24][25] The study revealed that even single point mutations had a significant effect on the enhancer expression pattern. Furthermore, the mutations affected multiple components of the pattern. This pleiotropic nature of the mutations was demonstrated when the emergence of novel salivary gland or mouth hook expression was linked with the nearly complete loss of the original embryonic expression pattern. Additionally, changes to the low-affinity Ultrabithorax binding sites resulted in pleiotropic effects modulating the timing, pattern intensity, and ectopic expression. The authors concluded that enhancers are densely encoded with regulatory information and enhancer mutations are usually pleiotropic. Other recent studies in the yellow spot enhancer[26] and the Sonic Hedgehog ZRS enhancer[27] also support this claim. These findings may even suggest that the underlying cis-regulatory logic of an enhancer may constrain its evolution,[12] a claim also made my Preger Ben-Noon et al.[15]
The E6 enhancer
The E6 enhancer is expressed in the dorsal and quaternary cells of Drosophila embryos, larvae, and in the pupal epidermis.[28] The E6 enhancer is one of the five enhancers that contributed to the loss of the larval dorsal trichomes in Drosophila sechellia.[15] The molecular mechanism for this loss of expression was resolved by Preger Ben-Noon et al.,[28] where sechellia-E6 consecutively accumulated mutations in activator sites for Arrowhead and Pannier and gained a binding site for the repressor Abrupt. These mutations contributed to a 46% decrease in total embryonic shavenbaby expression, and affected the pleiotropic expression in the pupal epidermis.[15]
The Z1.3 enhancer
The Z1.3 enhancer is a minimalized fragment of the Z enhancer, and drives expression in the embryonic quaternary cells, the larval pharynx and proventriculus, and the pupal epidermis. The Z1.3 enhancer contributed to an estimated 28% loss of total embryonic expression in Drosophila sechellia. However, unlike in E6, the mutations that affected the embryonic pattern of Z1.3 had no effect on its pleiotropic pupal epidermis expression. Preger Ben-Noon et al. further dissected the Z1.3 enhancer and were able to minimalize the pleiotropic activity into two separate enhancers: Z0.3 and Z1.3R.[15]
The DG3 enhancer
The DG3 enhancer is primarily expressed in the ventral embryonic epidermis along with E3N and 7H. In larvae, DG3 is expressed in the dorsal and ventral regions, in the pharynix, esophagus, and proventriculus, and in the pupal epidermis.[15] A closer look at the ventral nuclei reveals that the shavenbaby gene physically colocalizes with higher concentrations of the Ultrabithorax protein and its cofactor Homothorax.[29] Additionally, the Drosophila line Df(svb)108 contains a deletion in the DG2, DG3, and Z enhancers. Heat shocking these lines does induce a slight decrease in the number of ventral trichomes. A closer look at the nuclei of these individual cells reveals both lower quanitifiable levels of the shavenbaby transcript and weaker nuclear microenvironment interactions between the ventral enhancers . Interestingly, transcript levels and the microenvironment can be stabilized by crossing flies carrying the deletion with flies carrying an artificial BAC of the shavenbaby locus.[30] The studies from Tsai et al. reveals microenvironments and potentially transvection to be potential mechanisms for how redundant enhancers canalize gene expression.[29][30]
The 7H enhancer
The 7H enhancer drives expression in both the ventral and dorsal embryonic and larval epidermis, the larval pharynx, and the pupal epidermis. Deletion of the 7H enhancer results in a 38% decrease in total embryonic shavenbaby expression.[15] 7H, DG3, and E3N are the primary ventral enhancers in the embryo.[2]
Trichome formation
Shavenbaby activates over 150 different downstream targets to express actin-remodeling proteins to form the denticle.[3] Some of these factors include forked, shavenoid, singed, wasp, yellow, and miniature.[8] Activation of these target genes is also dependent on SoxNeuro, one of the regulators of shavenbaby. Together, SoxNeuro and Shavenbaby act cooperatively to shape the denticles.[10]
References
- ↑ 1.0 1.1 1.2 Payre, FranÇois; Vincent, Alain; Carreno, Sebastien (July 1999). "ovo/svb integrates Wingless and DER pathways to control epidermis differentiation" (in en). Nature 400 (6741): 271–275. doi:10.1038/22330. ISSN 1476-4687. PMID 10421370. Bibcode: 1999Natur.400..271P. https://www.nature.com/articles/22330.
- ↑ 2.0 2.1 2.2 2.3 "The structure and evolution of cis-regulatory regions: the shavenbaby story". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 368 (1632): 20130028. December 2013. doi:10.1098/rstb.2013.0028. PMID 24218640.
- ↑ 3.0 3.1 Menoret, Delphine; Santolini, Marc; Fernandes, Isabelle; Spokony, Rebecca; Zanet, Jennifer; Gonzalez, Ignacio; Latapie, Yvan; Ferrer, Pierre et al. (2013-08-23). "Genome-wide analyses of Shavenbaby target genes reveals distinct features of enhancer organization". Genome Biology 14 (8): R86. doi:10.1186/gb-2013-14-8-r86. ISSN 1474-760X. PMID 23972280.
- ↑ Delon, Isabelle; Chanut-Delalande, Hélène; Payre, François (2003-07-01). "The Ovo/Shavenbaby transcription factor specifies actin remodelling during epidermal differentiation in Drosophila" (in en). Mechanisms of Development 120 (7): 747–758. doi:10.1016/S0925-4773(03)00081-9. ISSN 0925-4773. PMID 12915226.
- ↑ Wieschaus, Eric; Nüsslein-Volhard, Christiane (2016-10-06). "The Heidelberg Screen for Pattern Mutants of Drosophila : A Personal Account" (in en). Annual Review of Cell and Developmental Biology 32 (1): 1–46. doi:10.1146/annurev-cellbio-113015-023138. ISSN 1081-0706. PMID 27501451.
- ↑ 6.0 6.1 Stern, David L.; Orgogozo, Virginie (2009-02-06). "Is Genetic Evolution Predictable?" (in EN). Science 323 (5915): 746–751. doi:10.1126/science.1158997. PMID 19197055. Bibcode: 2009Sci...323..746S.
- ↑ 7.0 7.1 7.2 Kittelmann, Sebastian; Preger-Ben Noon, Ella; McGregor, Alistair P.; Frankel, Nicolás (2021-08-01). "A complex gene regulatory architecture underlies the development and evolution of cuticle morphology in Drosophila" (in en). Current Opinion in Genetics & Development 69: 21–27. doi:10.1016/j.gde.2021.01.003. ISSN 0959-437X. PMID 33529925. https://www.sciencedirect.com/science/article/abs/pii/S0959437X21000046.
- ↑ 8.0 8.1 8.2 Serge, Chanut-Delalande, Hélène Fernandes, Isabelle Roch, Fernando Payre, François Plaza. Shavenbaby couples patterning to epidermal cell shape control.. OCLC 798361751. http://worldcat.org/oclc/798361751.
- ↑ 9.0 9.1 9.2 9.3 Lawrence., Stern, David (2011). Evolution, development, and the predictable genome. Roberts and Co. Publishers. ISBN 978-1-936221-01-1. OCLC 730443756. http://worldcat.org/oclc/730443756.
- ↑ 10.0 10.1 10.2 Rizzo, Nicholas P.; Bejsovec, Amy (2017-06-15). "SoxNeuro and Shavenbaby act cooperatively to shape denticles in the embryonic epidermis of Drosophila". Development 144 (12): 2248–2258. doi:10.1242/dev.150169. ISSN 0950-1991. PMID 28506986. PMC 5482994. https://doi.org/10.1242/dev.150169.
- ↑ 11.0 11.1 11.2 11.3 11.4 Crocker, Justin; Abe, Namiko; Rinaldi, Lucrezia; McGregor, Alistair P.; Frankel, Nicolás; Wang, Shu; Alsawadi, Ahmad; Valenti, Philippe et al. (January 2015). "Low Affinity Binding Site Clusters Confer Hox Specificity and Regulatory Robustness". Cell 160 (1–2): 191–203. doi:10.1016/j.cell.2014.11.041. ISSN 0092-8674. PMID 25557079.
- ↑ 12.0 12.1 12.2 12.3 12.4 Fuqua, Timothy; Jordan, Jeff; van Breugel, Maria Elize; Halavatyi, Aliaksandr; Tischer, Christian; Polidoro, Peter; Abe, Namiko; Tsai, Albert et al. (November 2020). "Dense and pleiotropic regulatory information in a developmental enhancer" (in en). Nature 587 (7833): 235–239. doi:10.1038/s41586-020-2816-5. ISSN 1476-4687. PMID 33057197. Bibcode: 2020Natur.587..235F.
- ↑ Long, Hannah K.; Prescott, Sara L.; Wysocka, Joanna (2016-11-17). "Ever-Changing Landscapes: Transcriptional Enhancers in Development and Evolution" (in en). Cell 167 (5): 1170–1187. doi:10.1016/j.cell.2016.09.018. ISSN 0092-8674. PMID 27863239.
- ↑ Stern, David L.; Frankel, Nicolás (2013-12-19). "The structure and evolution of cis -regulatory regions: the shavenbaby story". Philosophical Transactions of the Royal Society B: Biological Sciences 368 (1632): 20130028. doi:10.1098/rstb.2013.0028. ISSN 0962-8436. PMID 24218640. PMC 3826501. http://dx.doi.org/10.1098/rstb.2013.0028.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 Preger-Ben Noon, Ella; Sabarís, Gonzalo; Ortiz, Daniela M.; Sager, Jonathan; Liebowitz, Anna; Stern, David L.; Frankel, Nicolás (2018-03-13). "Comprehensive Analysis of a cis-Regulatory Region Reveals Pleiotropy in Enhancer Function". Cell Reports 22 (11): 3021–3031. doi:10.1016/j.celrep.2018.02.073. ISSN 2211-1247. PMID 29539428. https://pubmed.ncbi.nlm.nih.gov/29539428/.
- ↑ Barolo, Scott (February 2012). "Shadow enhancers: Frequently asked questions about distributed cis-regulatory information and enhancer redundancy". BioEssays 34 (2): 135–141. doi:10.1002/bies.201100121. ISSN 0265-9247. PMID 22083793.
- ↑ 17.0 17.1 Frankel, Nicolás; Davis, Gregory K.; Vargas, Diego; Wang, Shu; Payre, François; Stern, David L. (July 2010). "Phenotypic robustness conferred by apparently redundant transcriptional enhancers" (in en). Nature 466 (7305): 490–493. doi:10.1038/nature09158. ISSN 1476-4687. PMID 20512118. Bibcode: 2010Natur.466..490F.
- ↑ Osterwalder, Marco; Barozzi, Iros; Tissières, Virginie; Fukuda-Yuzawa, Yoko; Mannion, Brandon J.; Afzal, Sarah Y.; Lee, Elizabeth A.; Zhu, Yiwen et al. (February 2018). "Enhancer redundancy provides phenotypic robustness in mammalian development" (in en). Nature 554 (7691): 239–243. doi:10.1038/nature25461. ISSN 1476-4687. PMID 29420474. Bibcode: 2018Natur.554..239O.
- ↑ Wunderlich, Zeba; Bragdon, Meghan D.J.; Vincent, Ben J.; White, Jonathan A.; Estrada, Javier; DePace, Angela H. (March 2016). "Krüppel Expression Levels Are Maintained through Compensatory Evolution of Shadow Enhancers". Cell Reports 14 (12): 3030. doi:10.1016/j.celrep.2016.03.032. ISSN 2211-1247. PMID 27028762.
- ↑ Al Hayek, Sandy; Alsawadi, Ahmad; Kambris, Zakaria; Boquete, Jean-Philippe; Bohère, Jérôme; Immarigeon, Clément; Ronsin, Brice; Plaza, Serge et al. (2021-02-15). "Steroid-dependent switch of OvoL/Shavenbaby controls self-renewal versus differentiation of intestinal stem cells". The EMBO Journal 40 (4): e104347. doi:10.15252/embj.2019104347. ISSN 0261-4189. PMID 33372708.
- ↑ "Hox Genes in Development: The Hox Code | Learn Science at Scitable". https://www.nature.com/scitable/topicpage/hox-genes-in-development-the-hox-code-41402/.
- ↑ "Solving the Hox Specificity Paradox" (in en). https://www.hhmi.org/news/solving-hox-specificity-paradox.
- ↑ Farley, Emma K.; Olson, Katrina M.; Zhang, Wei; Rokhsar, Daniel S.; Levine, Michael S. (2016-06-07). "Syntax compensates for poor binding sites to encode tissue specificity of developmental enhancers". Proceedings of the National Academy of Sciences 113 (23): 6508–6513. doi:10.1073/pnas.1605085113. PMID 27155014. Bibcode: 2016PNAS..113.6508F.
- ↑ Fuqua, Timothy; Jordan, Jeff; van Breugel, Maria Elize; Halavatyi, Aliaksandr; Tischer, Christian; Polidoro, Peter; Abe, Namiko; Tsai, Albert et al. (November 2020). "Dense and pleiotropic regulatory information in a developmental enhancer" (in en). Nature 587 (7833): 235–239. doi:10.1038/s41586-020-2816-5. ISSN 1476-4687. PMID 33057197. Bibcode: 2020Natur.587..235F.
- ↑ Timothy, Fuqua; Jeff, Jordan; Aliaksandr, Halavatyi; Christian, Tischer; Kerstin, Richter; Justin, Crocker (2021-05-13). "An open-source semi-automated robotics pipeline for embryo immunohistochemistry" (in en). Scientific Reports 11 (1): 10314. doi:10.1038/s41598-021-89676-5. ISSN 2045-2322. PMID 33986394. Bibcode: 2021NatSR..1110314F.
- ↑ Poul, Yann Le; Xin, Yaqun; Ling, Liucong; Mühling, Bettina; Jaenichen, Rita; Hörl, David; Bunk, David; Harz, Hartmann et al. (December 2020). "Regulatory encoding of quantitative variation in spatial activity of a Drosophila enhancer" (in EN). Science Advances 6 (49). doi:10.1126/sciadv.abe2955. PMID 33268361. Bibcode: 2020SciA....6.2955L.
- ↑ Kvon, Evgeny Z.; Zhu, Yiwen; Kelman, Guy; Novak, Catherine S.; Plajzer-Frick, Ingrid; Kato, Momoe; Garvin, Tyler H.; Pham, Quan et al. (2020-03-19). "Comprehensive In Vivo Interrogation Reveals Phenotypic Impact of Human Enhancer Variants". Cell 180 (6): 1262–1271.e15. doi:10.1016/j.cell.2020.02.031. ISSN 1097-4172. PMID 32169219.
- ↑ 28.0 28.1 Preger-Ben Noon, Ella; Davis, Fred P.; Stern, David L. (2016-12-05). "Evolved Repression Overcomes Enhancer Robustness". Developmental Cell 39 (5): 572–584. doi:10.1016/j.devcel.2016.10.010. ISSN 1878-1551. PMID 27840106.
- ↑ 29.0 29.1 Tsai, Albert; Muthusamy, Anand K; Alves, Mariana RP; Lavis, Luke D; Singer, Robert H; Stern, David L; Crocker, Justin (2017-11-02). Arnosti, David N. ed. "Nuclear microenvironments modulate transcription from low-affinity enhancers". eLife 6: e28975. doi:10.7554/eLife.28975. ISSN 2050-084X. PMID 29095143.
- ↑ 30.0 30.1 Tsai, Albert; Alves, Mariana RP; Crocker, Justin (2019-07-11). Arnosti, David N; Tyler, Jessica K; DePace, Angela H et al.. eds. "Multi-enhancer transcriptional hubs confer phenotypic robustness". eLife 8: e45325. doi:10.7554/eLife.45325. ISSN 2050-084X. PMID 31294690.
 |