Biology:Superspreader
A superspreader is an unusually contagious organism infected with a disease. In the context of a human-borne illness, a superspreader is an individual who is more likely to infect others, compared with a typical infected person. Such superspreaders are of particular concern in epidemiology.
Some cases of superspreading conform to the 80/20 rule,[1] where approximately 20% of infected individuals are responsible for 80% of transmissions, although superspreading can still be said to occur when superspreaders account for a higher or lower percentage of transmissions.[2] In epidemics with such superspreader events (SSEV), the majority of individuals infect relatively few secondary contacts.[citation needed]
SSEVs are shaped by multiple factors including a decline in herd immunity, nosocomial infections, virulence, viral load, misdiagnosis, airflow dynamics, immune suppression, and co-infection with another pathogen.[3]
Children, who can superspread measles disease,[4] appear not to superspread SARS-CoV-2.[5] In fact, some children appear to have a form of immunity from the more recent disease.[6]
Defining a superspreader event
Although loose definitions of superspreader events exist, some effort has been made at defining what qualifies as a superspreader event (SSEV). Lloyd-Smith et al. (2005) define a protocol to identify a superspreader event as follows:[2]
- estimate the effective reproductive number, R, for the disease and population in question;
- construct a Poisson distribution with mean R, representing the expected range of Z due to stochasticity without individual variation;
- define an SSEV as any infected person who infects more than Z(n) others, where Z(n) is the nth percentile of the Poisson(R) distribution.
This protocol defines a 99th-percentile SSEV as a case which causes more infections than would occur in 99% of infectious histories in a homogeneous population.[2]
During the SARS-CoV-1 2002–2004 SARS outbreak from China, epidemiologists defined a superspreader as an individual with at least eight transmissions of the disease.[7]
Superspreaders may or may not show any symptoms of the disease.[3][8]
Writing in Quillette on 23 April 2020 and again a week later in the National Post, Jonathan Kay noticed that in the case of the SARS‑CoV‑2 virus outbreak from China which subsequently turned into the COVID-19 pandemic:[9][10]
| “ | Putting aside hospitals, private residences and old-age homes, almost all of these superspreader events (SSEVs) took place in the context of (1) parties, (2) face-to-face professional networking events and meetings, (3) religious gatherings, (4) sports events, (5) meat-processing facilities, (6) ships at sea, (7) singing groups, and, yes, (8) funerals. | ” |
Factors in transmission

Superspreaders have been identified who excrete a higher than normal number of pathogens during the time they are infectious. This causes their contacts to be exposed to higher viral/bacterial loads than would be seen in the contacts of non-superspreaders with the same duration of exposure.[11]
Basic reproductive number
The basic reproduction number R0 is the average number of secondary infections caused by a typical infective person in a totally susceptible population.[12] The basic reproductive number is found by multiplying the average number of contacts by the average probability that a susceptible individual will become infected, which is called the shedding potential.[2]
R0 = Number of contacts × Shedding potential
Individual reproductive number
The individual reproductive number represents the number of secondary infections caused by a specific individual during the time that individual is infectious. Some individuals have significantly higher than average individual reproductive numbers and are known as superspreaders. Through contact tracing, epidemiologists have identified superspreaders in measles, tuberculosis, rubella, monkeypox, smallpox, Ebola hemorrhagic fever and SARS.[2][13]
Co-infections with other pathogens
Studies have shown that men with HIV who are co-infected with at least one other sexually transmitted disease, such as gonorrhea, hepatitis C, and herpes simplex 2 virus, have a higher HIV shedding rate than men without co-infection. This shedding rate was calculated in men with similar HIV viral loads. Once treatment for the co-infection has been completed, the HIV shedding rate returns to levels comparable to men without co-infection.[14][15]
Lack of herd immunity
Herd immunity, or herd effect, refers to the indirect protection that immunized community members provide to non-immunized members in preventing the spread of contagious disease. The greater the number of immunized individuals, the less likely an outbreak can occur because there are fewer susceptible contacts. In epidemiology, herd immunity is known as a dependent happening because it influences transmission over time. As a pathogen that confers immunity to the survivors moves through a susceptible population, the number of susceptible contacts declines. Even if susceptible individuals remain, their contacts are likely to be immunized, preventing any further spread of the infection.[11][16] The proportion of immune individuals in a population above which a disease may no longer persist is the herd immunity threshold. Its value varies with the virulence of the disease, the efficacy of the vaccine, and the contact parameter for the population.[17] That is not to say that an outbreak can't occur, but it will be limited.[16][18][19]
Superspreaders during outbreaks
COVID-19 outbreak 2020
The South Korean spread of confirmed cases of SARS-CoV-2 infection jumped suddenly starting on 19–20 February 2020. On 19 February, the number of confirmed cases increased by 20. On 20 February, 58[20] or 70[21] new cases were confirmed, giving a total of 104 confirmed cases, according to the Centers for Disease Control and Prevention Korea (KCDC). According to Reuters , KCDC attributed the sudden jump to 70 cases linked to "Patient 31", who had participated in a gathering in Daegu at the Shincheonji Church of Jesus the Temple of the Tabernacle of the Testimony.[21] On 20 February, the streets of Daegu were empty in reaction to the Shincheonji outbreak. A resident described the reaction, stating "It's like someone dropped a bomb in the middle of the city. It looks like a zombie apocalypse."[21] On 21 February, the first death was reported.[22] According to the mayor of Daegu, the number of suspected cases as of 21 February is 544 among 4,400 examined followers of the church.[23] Later in the outbreak, in May, A 29-year-old man visited several Seoul nightclubs in one night and resulted in accumulated infections of at least 79 other people.[24]
In New York, a lawyer contracted the illness then spread it to at least twenty other individuals in his community in New Rochelle, creating a cluster of cases that quickly passed 100,[25] accounting for more than half of SARS2 coronavirus cases in the state during early March 2020.[26] For comparison, the basic reproduction number of the virus, which is the average number of additional people that a single case will infect without any preventative measures, is between 1.4 and 3.9.[27][28]
In India, Baldev Singh, a preacher, ignored self-quarantine after returning from Italy and Germany . As a result, 19 of his relatives have tested positive and 550 people had direct contact with him. Because of this, India's government fears an outbreak and quarantined on 27 March 2020 in the State of Punjab, 40,000 residents from 20 villages.[29]
On 11 May 2020, it came to light that a worker at a fish processing plant in Tema, Ghana is believed to have infected over 500 other people with COVID-19.[30]
As of 18 July 2020, more than one thousand suspected superspreading events had been logged, for example a cluster of 187 people who were infected after eating at a Harper's Restaurant and Brew Pub in East Lansing, Michigan.[31]
SARS outbreak 2003
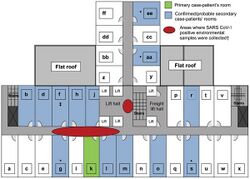
The first cases of SARS occurred in mid-November 2002 in the Guangdong Province of China. This was followed by an outbreak in Hong Kong in February 2003. A Guangdong Province doctor, Liu Jianlun, who had treated SARS cases there, had contracted the virus and was symptomatic. Despite his symptoms, he traveled to Hong Kong to attend a family wedding. He stayed on the ninth floor of the Metropole Hotel in Kowloon, infecting 16 other hotel guests also staying on that floor. The guests then traveled to Canada, Singapore, Taiwan, and Vietnam, spreading SARS to those locations and transmitting what became a global epidemic.[32]
In another case during this same outbreak, a 54-year-old male was admitted to a hospital with coronary heart disease, chronic kidney failure and type II diabetes mellitus. He had been in contact with a patient known to have SARS. Shortly after his admission he developed fever, cough, myalgia and sore throat. The admitting physician suspected SARS. The patient was transferred to another hospital for treatment of his coronary artery disease. While there, his SARS symptoms became more pronounced. Later, it was discovered he had transmitted SARS to 33 other patients in just two days. He was transferred back to the original hospital where he died of SARS.
The SARS outbreak was eventually contained, but not before it caused 8,273 cases and 775 deaths. Within two weeks of the original outbreak in Guangdong Province, SARS had spread to 29 countries.[33]
Measles outbreak 1989
Measles is a highly contagious, air-borne virus that reappears even among vaccinated populations. In one Finnish town in 1989, an explosive school-based outbreak resulted in 51 cases, several of whom had been previously vaccinated. One child alone infected 22 others. It was noted during this outbreak that when vaccinated siblings shared a bedroom with an infected sibling, seven out of nine became infected as well.[4]
Typhoid fever
Typhoid fever is a human-specific disease caused by the bacterium Salmonella typhi. It is highly contagious and becoming resistant to antibiotics.[34] S. typhi is susceptible to creating asymptomatic carriers. The most famous carriers are Mary Mallon, known as Typhoid Mary, from New York City, and Mr. N. the Milker, from Folkstone, England.[35] Both were active around the same time. Mallon infected 51 people from 1902 to 1909. Mr. N. infected more than 200 people over 14 years from 1901 to 1915. At the request of health officials, Mr. N. gave up working in food service. Mallon was at first also compliant, choosing other work – but eventually she returned to cooking and caused further outbreaks. She was involuntarily quarantined at Brothers Island in New York, where she stayed until she died in November 1938, aged 69.[36]
It has been found that Salmonella typhi persists in infected mice macrophages that have cycled from an inflammatory state to a non-inflammatory state. The bacteria remain and reproduce without causing further symptoms in the mice, and this helps to explain why carriers are asymptomatic.[37][38][39][40]
See also
- Compartmental models in epidemiology – Type of mathematical model used for infectious diseases
- Medicine:Disease outbreak – Sudden increase in occurrences of a disease
- Medicine:Index case – First documented patient in the population of an epidemiological investigation
- Finance:Pandemic – Widespread, often global, epidemic of severe infectious disease
References
- ↑ Galvani, Alison P.; May, Robert M. (2005). "Epidemiology: Dimensions of superspreading". Nature 438 (7066): 293–95. doi:10.1038/438293a. PMID 16292292. Bibcode: 2005Natur.438..293G.
- ↑ 2.0 2.1 2.2 2.3 2.4 Lloyd-Smith, JO; Schreiber, SJ; Kopp, PE; Getz, WM (2005). "Superspreading and the effect of individual variation on disease emergence". Nature 438 (7066): 355–59. doi:10.1038/nature04153. PMID 16292310. Bibcode: 2005Natur.438..355L.
- ↑ 3.0 3.1 Stein, Richard A. (2011). "Superspreaders in Infectious Disease". International Journal of Infectious Diseases 15 (8): 510–13. doi:10.1016/j.ijid.2010.06.020. PMID 21737332. "The minority of individuals who infect disproportionately more susceptible contacts, as compared to most individuals who infect few or no others, became known as super-spreaders, and their existence is deeply rooted in history: between 1900 and 1907, Typhoid Mary infected 51 individuals, three of whom died, even though she only had an asymptomatic infection.".
- ↑ 4.0 4.1 Paunio, Mikko; Peltola, Heikki; Davidkin, Irja; Virtanen, Martti; Heinonen, Olli P.; Valle, Martti (1998). "Explosive School-based Measles Outbreak Intense Exposure May Have Resulted in High Risk, Even among Revaccinees". Am J Epidemiol 148 (11): 1103–10. doi:10.1093/oxfordjournals.aje.a009588. PMID 9850133.
- ↑ Pfeffer, Amanda (4 May 2020). "Public health officials take seriously new research that children may not be superspreaders after all". CBC. https://www.cbc.ca/news/canada/ottawa/children-may-not-be-super-spreaders-afterall-new-research-suggests-1.5552099.
- ↑ "COVID-19 in schools – the experience in NSW". National Centre for Immunisation Research and Surveillance. 26 April 2020. http://ncirs.org.au/sites/default/files/2020-04/NCIRS%20NSW%20Schools%20COVID_Summary_FINAL%20public_26%20April%202020.pdf.
- ↑ Shen, Zhuang; Ning, Fang; Zhou, Weigong; He, Xiong; Lin, Changying; Chin, Daniel P.; Zhu, Zonghan; Schuchat, Anne (2004). "Superspreading SARS Events, Beijing, 2003". Emerging Infectious Diseases 10 (2): 256–260. doi:10.3201/eid1002.030732. PMID 15030693.
- ↑ Cory, David C. Wiley, Amy C. (2013). Encyclopedia of School Health. Los Angeles, CA: Sage. ISBN 978-1412996006. "Historically, one of the most famous examples of super-spreading was that of Mary Mallon, better known as Typhoid Mary, who infected many contacts, several of whom died, through food she prepared and consequently contaminated, even thought she did not show symptoms."
- ↑ Kay, Jonathan (30 April 2020). "It's not the size of the event, but the behaviour that matters". National Post, a division of Postmedia Network Inc. https://nationalpost.com/opinion/jonathan-kay-on-covid-19-its-not-the-size-of-the-event-but-behaviour-that-matters?video_autoplay=true.
- ↑ Kay, Jonathan (23 April 2020). "COVID-19 Superspreader Events in 28 Countries: Critical Patterns and Lessons". Quillette Pty Ltd. https://quillette.com/2020/04/23/covid-19-superspreader-events-in-28-countries-critical-patterns-and-lessons/.
- ↑ 11.0 11.1 Kenneth J. Rothman, Sander Greenland, and Timothy L. Lash. Modern Epidemiology, 3rd Edition. 2008. p. 561. Lippincott, Williams & Wilkins. Philadelphia.
- ↑ Galvani, Alison P.; May, Robert M. (17 November 2005). "Epidemiology: Dimensions of super-spreading". Nature 438 (7066): 239–95. doi:10.1038/438293a. PMID 16292292. Bibcode: 2005Natur.438..293G.
- ↑ De Serres, GExpression error: Unrecognized word "etal". (2013). "Largest measles epidemic in North America in a decade–Quebec, Canada, 2011: contribution of susceptibility, serendipity, and superspreading events". J Infect Dis 207 (6): 990–98. doi:10.1093/infdis/jis923. PMID 23264672.
- ↑ Cohen, M.S. HoffmanExpression error: Unrecognized word "etal". (1997). "Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group". Lancet 349 (9069): 1868–73. doi:10.1016/s0140-6736(97)02190-9. PMID 9217758.
- ↑ Winter, AJ; Taylor, S. Workman J.; White, D.; Ross, JD.; Swan, AV; Pillay, D. (1999). "Asymptomatic urethritis and detection of HIV-1 RNA in seminal plasma". Sex Transm Infect 75 (261): 261–63. doi:10.1136/sti.75.4.261. PMID 10615314.
- ↑ 16.0 16.1 Fine, P (1993). "Herd immunity: history, theory, practice". Epidemiol Rev 15 (2): 265–302. doi:10.1093/oxfordjournals.epirev.a036121. PMID 8174658.
- ↑ "Chapter 4: Cost-Effective Strategies for the Excess Burden of Disease in Developing Countries
Section: Vaccine-preventable Diseases". Priorities in Health: Disease Control Priorities Companion Volume. World Bank Publications. 2006. ISBN 0-8213-6260-7. https://www.ncbi.nlm.nih.gov/books/NBK10258/#A151. - ↑ Yeung, L. F.; Lurie, P.; Dayan, G.; Eduardo, E.; Britz, P. H.; Redd, S. B.; Papania, M. J.; Seward, J. F. (2005). "A Limited Measles Outbreak in a Highly Vaccinated US Boarding School". Pediatrics 116 (6): 1287–1291. doi:10.1542/peds.2004-2718. PMID 16322148.
- ↑ Fine, P.; Eames, K.; Heymann, D. L. (2011). ""Herd Immunity": A Rough Guide". Clinical Infectious Diseases 52 (7): 911–916. doi:10.1093/cid/cir007. PMID 21427399.
- ↑ "코로나19 확진자 104명...31번 환자 연관 신천지교회 대남병원서만 확진자 58명, 1명 사망" (in ko). 글로벌경제신문. 20 February 2020. http://m.getnews.co.kr/view.php?ud=2020022021135849202aaae0046f_16. Retrieved 16 March 2020.
- ↑ 21.0 21.1 21.2 Shin, Hyonhee; Cha, Sangmi (2020-02-20). "'Like a zombie apocalypse': Residents on edge as coronavirus cases surge in South Korea". Thomson Reuters. Archived from the original on 2020-02-20. https://archive.today/D1K5i. Retrieved 2020-02-20.
- ↑ "South Korea reports first coronavirus death as infections linked to church rise" (in en). https://www.nbcnews.com/health/health-news/south-korea-reports-first-coronavirus-death-infections-linked-church-rise-n1139821.
- ↑ "신천지 관련 확진자 76명으로 늘어...대구 교인 의심자만 544명". Chosun.com. 21 February 2020. https://news.chosun.com/site/data/html_dir/2020/02/21/2020022101558.html.
- ↑ Park Han-na (11 May 2020). "Itaewon cluster caseload rises to 79: Nation adds 35 new cases, 20 in Seoul". Archived from the original on 16 May 2020. https://web.archive.org/web/20200516111911/http://nwww.koreaherald.com/view.php?ud=20200511000575.
- ↑ CNN, Sheena Jones and Christina Maxouris. "New York officials traced more than 50 coronavirus cases back to one attorney". https://www.cnn.com/2020/03/11/us/new-rochelle-attorney-containment-area/index.html. Retrieved 13 March 2020.
- ↑ CNN, Eric Levenson and Kristina Sgueglia. "New York creates 'containment zone' around cluster of coronavirus cases in New Rochelle". https://www.cnn.com/2020/03/10/us/new-rochelle-coronavirus/index.html. Retrieved 13 March 2020.
- ↑ "Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia". The New England Journal of Medicine 382 (13): 1199–1207. January 2020. doi:10.1056/NEJMoa2001316. PMID 31995857.
- ↑ "Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020". Euro Surveillance 25 (4). January 2020. doi:10.2807/1560-7917.ES.2020.25.4.2000058. PMID 32019669.
- ↑ "India 'super spreader' quarantines 40,000 people" (in en-GB). BBC News. 2020-03-27. https://www.bbc.com/news/world-asia-india-52061915.
- ↑ CNN, Bukola Adebayo. "A worker infected 533 others with coronavirus at a factory in Ghana, president says". https://www.cnn.com/2020/05/11/africa/ghana-factory-coronavirus-infection-intl/index.html. Retrieved 11 May 2020. "Hundreds of factory workers at a fish processing plant in Ghana have tested positive for the coronavirus, the country's president Nana Akufo-Addo said. All 533 of them contracted the virus from one worker at the factory in the port city of Tema, the president said in his public address to the nation Sunday."
- ↑ Cha, Ariana. "'Superspreading' events, triggered by people who may not even know they are infected, propel coronavirus pandemic". https://www.washingtonpost.com/health/2020/07/18/coronavirus-superspreading-events-drive-pandemic/. Retrieved 18 July 2020.
- ↑ "How SARS changed the world in less than six months". Archived from the original on April 5, 2012. https://web.archive.org/web/20120405125444/http://www.scielosp.org/pdf/bwho/v81n8/v81n8a14.pdf. Retrieved February 4, 2016.
- ↑ Shen, Zhuang; Fang Ning (February 2004). "Superspreading SARS Events, Beijing 2003". Emerging Infectious Diseases 10 (2): 256–60. doi:10.3201/eid1002.030732. PMID 15030693.
- ↑ Highfield, Roger (28 November 2006). "Typhoid is with us to stay". The Daily Telegraph. https://www.telegraph.co.uk/health/healthnews/3345526/Typhoid-is-with-us-to-stay.html. Retrieved 2015-03-03.
- ↑ Mortimer, Philip P. (1999). "Mr N the milker, and Dr Koch's concept of the healthy carrier". The Lancet 353 (9161): 1354–1356. doi:10.1016/S0140-6736(98)11315-6. PMID 10218549.
- ↑ Marr, John S. (1999). "Typhoid Mary". The Lancet 353 (9165): 1714. doi:10.1016/S0140-6736(05)77031-8. PMID 10335825.
- ↑ Ng, Tessie M.; Monack, Denise M. (2013). "Revisiting Caspase-11 Function in Host Defense". Cell Host & Microbe 14 (1): 9–14. doi:10.1016/j.chom.2013.06.009. PMID 23870309.
- ↑ Eisele, Nicholas A.; Ruby, Thomas; Jacobson, Amanda; Manzanillo, Paolo S.; Cox, Jeffery S.; Lam, Lilian; Mukundan, Lata; Chawla, Ajay et al. (2013). "Salmonella Require the Fatty Acid Regulator PPARδ for the Establishment of a Metabolic Environment Essential for Long-Term Persistence". Cell Host & Microbe 14 (2): 171–182. doi:10.1016/j.chom.2013.07.010. PMID 23954156.
- ↑ Geoffrey Mohan (August 14, 2013). "Typhoid Mary case may be cracked, a century later". Los Angeles Times. http://www.latimes.com/science/sciencenow/la-sci-sn-typhoid-mary-20130814-story.html. Retrieved 2015-03-03.
- ↑ Donald G. McNeil Jr. (August 26, 2013). "Bacteria study offers clues to Typhoid Mary mystery". The New York Times. https://www.nytimes.com/2013/08/27/health/bacteria-study-offers-clues-to-typhoid-mary-mystery.html?_r=0. Retrieved 2015-03-03.
External links
- World Health Organisation (WHO) – authoritative source of information about global health issues
- Past pandemics that ravaged Europe at the BBC
- Influenza pandemic phases at the US Center for Disease Control (CDC)
- European Centre for Disease Prevention and Control (ECDC)
- TED-Education video – How pandemics spread
