Biology:Tripartite ATP-independent periplasmic transporter
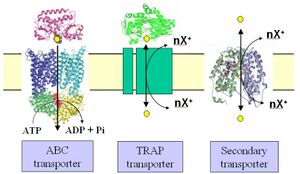
Tripartite ATP-independent periplasmic transporters (TRAP transporters) are a large family of solute transporters found in bacteria and archaea, but not in eukaryotes, that appear to be specific for the uptake of organic acids or related molecules containing a carboxylate or sulfonate group. They are unique in that they utilize a substrate binding protein (SBP) in combination with a secondary transporter.
History
TRAP transporters were discovered in the laboratory of Prof. David J. Kelly at the University of Sheffield, UK. His group were working on the mechanism used by the photosynthetic bacterium Rhodobacter capsulatus to take up certain dicarboxylic acids. They characterised a binding protein component (DctP) of a transporter that recognized these compounds, which they assumed would form part of a typical ABC transporter, but when they sequenced the genes surrounding dctP they found two other genes encoding integral membrane proteins, dctQ and dctM, but no genes encoding components of an ABC transporter.[1] They further showed that uptake of the same dicarboxylates was independent of ATP and that uptake required an electrochemical ion gradient, making this a unique binding protein-dependent secondary transporter.[1]
Since these early studies, it has become clear that TRAP transporters are present in many bacteria and archaea,[2] with many bacterial having multiple TRAP transporters, some having over 20 different systems.[3]
Substrates
To date, most substrates for TRAP transporters contain a common feature which is that they are organic acids.[4] This includes C4-dicarboxylates such as succinate, malate and fumarate,[1] keto-acids such as pyruvate and alpha-ketobutyrate[5][6] and the sugar acid, N-acetyl neuraminic acid (or sialic acid).[7] Other substrates include the compatible solute ectoine and hydroxyectoine and pyroglutamate.[4]
Composition
All known TRAP transporters contain 3 protein domains. These are the solute binding protein (the SBP), the small membrane protein domain and the large membrane protein domain. Following the nomenclature for the first characterized TRAP transporter, DctPQM, these subunits are usually named P, Q and M respectively.[4] Around 10% of TRAP transporters have natural genetic fusions between the two membrane protein components, and in the one well studied example of this in the sialic acid specific TRAP transporter from Haemophilus influenzae the fused gene has been named siaQM.
Mechanism
By using an SBP, TRAP transporters share some similarity to ABC transporters in that the substrate for the transporter is initially recognized outside of the cytoplasmic membrane. In Gram-negative bacteria, the SBP is usually free in the periplasm and expressed at relatively high levels compared to the membrane domains.[1] In Gram positive bacteria and archaea, the SBP is tethered to the cytoplasmic membrane. In both types of systems the SBP binds to substrate, usually with low micromolar affinity,[4] which causes a significant conformation change in the protein, akin to a Venus flytrap closing. The trapped substrate is then delivered to the membrane domains of the transporter, where the electrochemical ion gradient is somehow exploited to open the SBP, extract the substrate and catalyse its movement across the membrane. For the SiaPQM TRAP transporter which has been studied in a fully reconstituted in vitro form, uptake uses a Na+ gradient and not proton gradient to drive uptake.[8] The SiaPQM systems also exhibits unique properties for a secondary transporter in that it cannot catalyse bidirectional transport as the SBP imposes that movement is only in the direction of uptake into the cell.[8]
Structure
Substrate binding protein (SBP)
Following the first structure of a TRAP SBP in 2005,[9] there are now over 10 different structures available.[10][11][12] They all have very similar overall structures, with two globular domains linked by a hinge. The substrate binding site is formed by both the domains which enclose the substrate. A highly conserved arginine residue in the TRAP SBPs forms a salt bridge with a carboxylate group on the substrate, which is important for substrate recognition.[10]
Membrane subunits
The first structure of the membrane domains was solved for the sialic acid transporter from Haemophilus influenzae.[13] [14] Confirmed by another TRAP transporter structure,[15] the structures reveal a monomeric elevator-like transport mechanism for TRAP transporter. While the larger M-subunit forms the stator- and elevator-domain, the Q-subunit seems to enlarge and stabilize the stator-domain of the monomeric transporter. Current models suggest a binding of the SBP with both functional parts of the transporter, the stator- and elevator domain, and show a matched conformational coupling between the inward-open and outward-open state of the membrane subunits to the opened and closed state of the SBP.[13] [14]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 Forward J.A.; Behrendt M.C.; Wyborn N.R.; Cross R.; Kelly D.J. (1997). "TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQM genes of Rhodobacter capsulatus and by homologs in diverse gram-negative bacteria". J. Bacteriol. 179 (17): 5482–5493. doi:10.1128/jb.179.17.5482-5493.1997. PMID 9287004.
- ↑ Rabus R.; Jack D.L.; Kelly D.J.; Saier M.H. Jr. (1999). "TRAP transporters: an ancient family of extracytoplasmic solute-receptor-dependent secondary active transporters". Microbiology 145 (12): 3431–3445. doi:10.1099/00221287-145-12-3431. PMID 10627041.
- ↑ Mulligan C.; Kelly D.J.; Thomas G.H. (2007). "Tripartite ATP-independent periplasmic transporters: application of a relational database for genome-wide analysis of transporter gene frequency and organization". J. Mol. Microbiol. Biotechnol. 12 (3–4): 218–226. doi:10.1159/000099643. PMID 17587870.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 Mulligan C.; Fischer M.; Thomas G. (2010). "Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea". FEMS Microbiol. Rev. 35 (1): 68–86. doi:10.1111/j.1574-6976.2010.00236.x. PMID 20584082.
- ↑ "Novel ligands for the extracellular solute receptors of two bacterial TRAP transporters". Microbiology 152 (2): 187–198. 2006. doi:10.1099/mic.0.28334-0. PMID 16385129.
- ↑ "A TRAP transporter for pyruvate and other monocarboxylate 2-oxoacids in the cyanobacterium Anabaena sp. strain PCC 7120". J. Bacteriol. 192 (22): 6089–6092. 2010. doi:10.1128/JB.00982-10. PMID 20851902.
- ↑ "Sialic acid transport in Haemophilus influenzae is essential for lipopolysaccharide sialylation and serum resistance and is dependent on a novel tripartite ATP-independent periplasmic transporter". Mol. Microbiol. 58 (4): 1173–1185. 2005. doi:10.1111/j.1365-2958.2005.04901.x. PMID 16262798.
- ↑ Jump up to: 8.0 8.1 Mulligan C.; Geertsma E.R.; Severi E.; Kelly D.J.; Poolman B.; Thomas G.H. (2009). "The substrate-binding protein imposes directionality on an electrochemical sodium gradient-driven TRAP transporter". Proc. Natl. Acad. Sci. USA 106 (6): 1778–1783. doi:10.1073/pnas.0809979106. PMID 19179287. Bibcode: 2009PNAS..106.1778M.
- ↑ Müller A.; Severi E.; Mulligan C.; Watts A.G.; Kelly D.J.; Wilson K.S.; Wilkinson A.J.; Thomas G.H. (2006). "Conservation of structure and mechanism in primary and secondary transporters exemplified by SiaP, a sialic acid binding virulence factor from Haemophilus influenzae". J. Biol. Chem. 281 (31): 22212–22222. doi:10.1074/jbc.M603463200. PMID 16702222. http://eprints.whiterose.ac.uk/2587/1/thomasgh1_muller_in_press.pdf.
- ↑ Jump up to: 10.0 10.1 Johnston J.W.; Coussens N.P.; Allen S.; Houtman J.C.; Turner K.H.; Zaleski A.; Ramaswamy S.; Gibson B.W. et al. (2008). "Characterization of the N-acetyl-5-neuraminic acid-binding site of the extracytoplasmic solute receptor (SiaP) of nontypeable Haemophilus influenzae strain 2019". J. Biol. Chem. 283 (2): 855–865. doi:10.1074/jbc.M706603200. PMID 17947229.
- ↑ Gonin S.; Arnoux P.; Pierru B.; Lavergne J.; Alonso B.; Sabaty M.; Pignol D. (2007). "Crystal structures of an Extracytoplasmic Solute Receptor from a TRAP transporter in its open and closed forms reveal a helix-swapped dimer requiring a cation for alpha-keto acid binding". BMC Struct. Biol. 7: 11. doi:10.1186/1472-6807-7-11. PMID 17362499.
- ↑ "Caught in a TRAP: substrate-binding proteins in secondary transport". Trends Microbiol. 18 (10): 471–478. 2010. doi:10.1016/j.tim.2010.06.009. PMID 20656493.
- ↑ Jump up to: 13.0 13.1 Hagelueken, Gregor; Peter, Martin F. (3 December 2021). "The structure of HiSiaQM defines the architecture of tripartite ATP-independent periplasmic (TRAP) transporters". bioRxiv. doi:10.1101/2021.12.03.471092.
- ↑ Jump up to: 14.0 14.1 Peter, Martin F.; Hagelueken, Gregor (December 2022). "Structural and mechanistic analysis of a tripartite ATP-independent periplasmic TRAP transporter". Nature Communications 13 (1): 4471. doi:10.1038/s41467-022-31907-y. PMID 35927235. Bibcode: 2022NatCo..13.4471P.
- ↑ Renwick C.J. Dobson, James S. Davies (14 February 2022). "Structure and mechanism of the tripartite ATP-independent periplasmic (TRAP) transporter". bioRxiv. doi:10.1101/2022.02.13.480285.
External links
- [1] The lab page of Prof. David Kelly, University of Sheffield, UK
- [2] The lab page of Dr. Gavin Thomas, University of York, UK.
 |