Biology:USP27X
The ubiquitin carboxyl-terminal hydrolase 27 (EC 3.4.19.12), also known as deubiquitinating enzyme 27, ubiquitin thioesterase 27 and USP27X,[1] is a deubiquitinating enzyme which is mainly characterized for cleaving ubiquitin (Ub) from proteins and other molecules. Ubiquitin binds to proteins so as to regulate the degradation of them via the proteasome and lysosome among many other functions.[2]
USP27X is encoded by the ubiquitin specific peptidase 27 X-linked gene, found in chromosome X in region Xp11.23.[3] It is mainly found in the cytosol and nucleus of cells, as well as the nucleoplasm and in vesicles. It is an intracellular protein found in many human tissues with low blood cell specificity.[4]

DUBs belong to the superfamily of proteases enzymes. Proteases can be classified into five different classes depending on the mechanism of catalysis: aspartic, metallo, serine, threonine and cystein proteases.[5]
Cysteine proteases comprise ubiquitin C-terminal hydrolases (UCHs), Machada Josepchin domain proteases (MJDs), ovarian tumour proteases (OTU) and ubiquitin-specific proteases (USPs). USP27X is included in the ubiquitin-specific proteases enzymes.[5]
| Ec | 3.4.19.12 |
| Taxonomic identifier
(assigned by the NCBI) |
9606 |
| Taxonomic lineage | eukaryota → metazoa → chordata → craniata → vertebrata → euteleostomi → mammalia → eutheria → euarchontoglires → primates → haplorrhini → catarrhini → homonidae → homo |
| Length | 1574bp |
| Mass | 49.6299 kDa |
| Gene Location
(in humans) |
|
| Cellular location | nucleus, cytoplasm |
| Databases | accession ID |
| HumanCyc | HS17606 |
| UniProt, nextProt | A6NNY8 |
| HGNC | HGNC:13486 |
| proteomics DB | A6NNY8 |
| biogrid | 133302 |
Protein structure
Deubiquitinating enzymes contain a conserved catalytic domain surrounded by other subdomains, some of which are known to contribute to target recognition, protein-protein interactions and location domains.[7][8] Specifically, USP domains are characterized for having interspersed points with insertions in the catalytic domain.[8] This insertions have the capacity of folding into independent domains that can be involved in the regulation of the enzyme's activity.[9]

All USP domains, including USP27X, can be divided into six conserved boxes that are mapped onto the USP domain core structure. These structures have been identified through multiple sequence alignment of the USP catalytic cores. Box 1 is known to contain the catalytic cysteine (Cys) residue, box 5 contains the catalytic histidine (His) residue, box 6 contains the catalytic asparagine (Asn)/ aspartate (Asp) residue. Box 3 and box 4 can vary its content although in most human USP enzyme domains each contain a conserved Cys-X-X-Cys motif, which is commonly associated with a zinc-binding site.[8]
USP27X has a length of 438 aminoacids. The residues that play a critical role in the active site can be found in position 87, in which resides the nucleophilic residue, and position 380 in which the proton acceptor is located.[1]
The number of aminoacids in USPs catalytic domain can vary from 295 and 850 residues. This variations suggest that either different subclasses of USP domains exist (variations of a catalyc fold) or that insertions are incorporated into a similar catalytic fold.
Function
As previously said USP27X belongs to deubiquitinase family, thus its main function is catalyzing the removal of ubiquitin from substrates (cleaving the bond between the last aminoacid of ubiquitin, glycine 76, and a lysine residue on the substrate), the reversal process of ubiquitination, regulating the fate of ubiquitin-conjugated proteins.[10]
Based on its conserved catalytic domains and its mechanism of catalysis, deubiquitinating enzymes are classified in the group of cysteine proteases. These enzymes use catalytic triads (groups of three aminoacids) to catalize the hydrolysis of the amine bonds between the ubiquitin and the substrate.[2] USP27X's catalytic triad is constituted by a cysteine, a histidine, and a third residue, which tends to be an asparagine.[11]
The ubiquitin is linked to the polypeptide molecule by an isopeptide bond between the carboxyl group (COO−) of ubiquitin's last glycine and the epsilon-amino group (ε-NH3+) of the substrate's lysine. The Asp functions to polarize and orient the imidazolium ring of the His, while the His gains a proton desprotonating the thiol group of the adjacent Cys. This Cys attacks on the isopeptide carbonyl carbon, breaking carbon's double bond with oxygen and converting it into a simple bond.[12] At the same time, the His gives its proton to the lysine epsilon-amino group, forming a bond between them. The isopeptide bond is broken and the carbonyl group (double bond) remade. The result of the reaction is the release of the target protein from histidine and the formation of a covalent intermediate with the Ub moiety since the bond between the thiol group from the cysteine and the carbon is broken and a new radical binds to the carboxyl group. Finally, a reaction of this intermediate with a water molecule results in the release of the free enzyme and Ub molecule.[13]
The Ub-proteasome system is the fundamental regulatory mechanism of protein stability, quality, and abundance, so USP27X plays a key role in a great number of processes regulated by this system, such as Snail 1 stabilization in pancreatic and breast cancer, resistance to chemotherapeutic agents on cancerous cells, e. g., Cisplatin, and it also intervenes on the regulation of Bim in melanoma and non-small cell lung cancer cells.[14]
Clinical relevance
USP27X deubiquitinating action has been discovered to regulate the presence of multiple proteins related to various human cancers, as well as affecting cancerous cells' resistance to chemotherapeutic agents.
Regulation of Snail1 in pancreatic and breast cancer
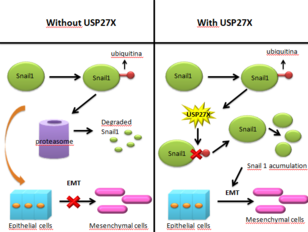
Cancerous cells gain the ability to migrate towards other parts of the body and to invade other tissues through what is called epithelial-mesenchymal transition. This process consists of a series of biochemical changes that transform an epithelial polarized cancerous cell into a cell with a mesenchymal phenotype. This new phenotype gives the cell an increased ability to migrate and invade other tissues, while it also decreases apoptotic activity. Therefore, EMT allows the cancerous cell to abandon the epithelial layer in which it originated, and migrate to other tissues. This is how metastasis in epithelial cancer begins.[15] EMT is controlled by various mesenchymal markers, amongst them Snail1, a protein also necessary in the activation of cancer associated fibroblasts (CAF). In a normal epithelial cell, Snail1 is continuously degraded by proteasomes, due to its previous ubiquitination. However, in cancer cells and CAFs, Snail1 degradation is prevented. One of the mechanisms that cancerous cells use to do so is USP27X. This protein deubiquitinates Snail1, meaning it cannot be degraded, therefore causing it to stabilize and to activate EMT, allowing metastasis. In cancerous cells, USP27X is regulated by TGFβ, and, summarizing, it causes the stabilization of Snail1 and other mesenchymal markers, leading to EMT. Although USP27X is not the only deubiquitinase that affects Snail1, it is one of the most effective and it has been proved to directly increase Snail1 presence by preventing its degradation. USP27X mechanism was observed in breast and pancreatic cancers, therefore opening a new door for treatments. Inhibition of USP27X would cause Snail1 to be ubiquitinated and then degraded, and therefore it would impair EMT.[16]
Influence on cancerous cells' resistance to chemotherapeutic agents
Snail1 has also been shown to increase the resistance of cancerous cells to certain chemotherapeutic agents, such as Cisplatin. When treated with Cisplatin, the amount of Snail1 increased, and this made the cells more resistant. However, when cisplatin was used in USP27X KO cells (cells without USP27X presence), Snail1 wasn't upregulated. This suggests that, after treatment with Cisplatin, Snail1 requires USP27X in order to increase its presence and make cells resistant. Therefore, inhibition of USP27X would impair Snail1 stabilization, leading to an increase in cancerous cells’ sensitivity to Cisplatin.[16]
Regulation of Bim in melanoma and non-small cell lung cancer cells
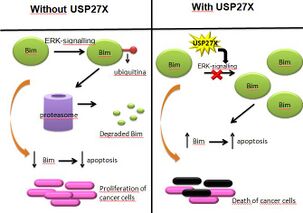
Bim is one of the proteins that induces apoptosis, and its depletion has been observed in multiple human tumours, meaning that cancer cells have mechanisms to avoid Bim expression or to degrade it, therefore annulling its apoptotic effects.
In some cancers, Bim is downregulated thanks to ERK-signaling.[17] Phosphorylation and ubiquitination of Bim dependent on ERK caused Bim to be degraded in proteasomes. However, presence of USP27X reduced ERK deubiquitination of BIM, causing Bim to stabilize and therefore increasing its apoptotic effects. This process has been proved in melanoma and non-small cell lung cancer (nsclc) cells.
Accordingly, we can conclude that USP27X can act as a tumour suppressor by inhibiting ERK-signalling, causing the deubiquitination and consequent stabilization of Bim, allowing it to induce apoptosis in cancer cells that use ERK-signalling.[18]
X-linked cognitive disorder
A relationship has been established between X-linked intellectual disability and certain mutations in the USP27X gene; specifically, an error in the reading of this gene causes a premature stop codon in the protein USP27X. Not much is known about the function of the USP27X protein yet, but its gene was found in mouse serotonin neurons, and that might mean that it is important for the serotonergetic function. USP27X interacts with USP22 which interacts with the KIF7 gene, which signals pathways involved in early development, cell growth and cell specialisation.[19][20] Besides, other proteins from the same peptidase C19 family such as the USP9X have been linked to several neurologic disorders.[19]
References
- ↑ 1.0 1.1 1.2 "USP27X - Ubiquitin carboxyl-terminal hydrolase 27 - Homo sapiens (Human) - USP27X gene & protein". https://www.uniprot.org/uniprot/A6NNY8.
- ↑ 2.0 2.1 "Deubiquitinating enzyme", Wikipedia, 2019-08-23, https://en.wikipedia.org/w/index.php?title=Deubiquitinating_enzyme&oldid=912173559, retrieved 2019-10-12
- ↑ 3.0 3.1 "Human Gene Database". https://www.genecards.org/cgi-bin/carddisp.pl?gene=USP27X. Retrieved 2019-10-12.
- ↑ "USP27X". https://www.proteinatlas.org/ENSG00000273820-USP27X/cell. Retrieved 2019-10-13.
- ↑ 5.0 5.1 Nijman, Sebastian M. B.; Luna-Vargas, Mark P. A.; Velds, Arno; Brummelkamp, Thijn R.; Dirac, Annette M. G.; Sixma, Titia K.; Bernards, René (2005-12-02). "A genomic and functional inventory of deubiquitinating enzymes". Cell 123 (5): 773–786. doi:10.1016/j.cell.2005.11.007. ISSN 0092-8674. PMID 16325574.
- ↑ "proteomic DB". https://www.proteomicsdb.org/proteomicsdb/#protein/proteinDetails/1647/summary.
- ↑ de Jong, Rob N.; Ab, Eiso; Diercks, Tammo; Truffault, Vincent; Daniëls, Mark; Kaptein, Robert; Folkers, Gert E. (2006-02-24). "Solution structure of the human ubiquitin-specific protease 15 DUSP domain". The Journal of Biological Chemistry 281 (8): 5026–5031. doi:10.1074/jbc.M510993200. ISSN 0021-9258. PMID 16298993.
- ↑ 8.0 8.1 8.2 Ye, Yu; Scheel, Hartmut; Hofmann, Kay; Komander, David (2009-11-12). "Dissection of USP catalytic domains reveals five common insertion points". Molecular BioSystems 5 (12): 1797–1808. doi:10.1039/B907669G. ISSN 1742-2051. PMID 19734957.
- ↑ "interpro7-client". https://www.ebi.ac.uk/interpro/entry/profile/PS50235/.
- ↑ Pickart, Cecile M. (June 2001). "Mechanisms Underlying Ubiquitination". Annual Review of Biochemistry 70 (1): 503–533. doi:10.1146/annurev.biochem.70.1.503. ISSN 0066-4154. PMID 11395416.
- ↑ López‐Iglesias, María; Gotor‐Fernández, Vicente (2015). "Recent Advances in Biocatalytic Promiscuity: Hydrolase-Catalyzed Reactions for Nonconventional Transformations". The Chemical Record 15 (4): 743–759. doi:10.1002/tcr.201500008. ISSN 1528-0691. PMID 26147872.
- ↑ "Reactome | Deubiquitination". https://reactome.org/content/detail/R-HSA-5688426.
- ↑ Nijman, Sebastian M. B.; Luna-Vargas, Mark P. A.; Velds, Arno; Brummelkamp, Thijn R.; Dirac, Annette M. G.; Sixma, Titia K.; Bernards, René (2005-12-02). "A Genomic and Functional Inventory of Deubiquitinating Enzymes" (in English). Cell 123 (5): 773–786. doi:10.1016/j.cell.2005.11.007. ISSN 0092-8674. PMID 16325574.
- ↑ Kaushal, Kamini; Antao, Ainsley Mike; Kim, Kye-Seong; Ramakrishna, Suresh (2018-12-01). "Deubiquitinating enzymes in cancer stem cells: functions and targeted inhibition for cancer therapy". Drug Discovery Today 23 (12): 1974–1982. doi:10.1016/j.drudis.2018.05.035. ISSN 1359-6446. PMID 29864528.
- ↑ Kalluri, Raghu; Weinberg, Robert A. (2009-06-01). "The basics of epithelial-mesenchymal transition". The Journal of Clinical Investigation 119 (6): 1420–1428. doi:10.1172/JCI39104. ISSN 0021-9738. PMID 19487818.
- ↑ 16.0 16.1 Lambies, Guillem; Miceli, Martina; Martínez-Guillamon, Catalina; Olivera-Salguero, Rubén; Peña, Raúl; Frías, Carolina-Paola; Calderón, Irene; Atanassov, Boyko S. et al. (1 Jan 2019). "TGFβ-Activated USP27X Deubiquitinase Regulates Cell Migration and Chemoresistance via Stabilization of Snail1". Cancer Research 79 (1): 33–46. doi:10.1158/0008-5472.CAN-18-0753. ISSN 1538-7445. PMID 30341066.
- ↑ Wan, Paul T. C; Garnett, Mathew J; Roe, S. Mark; Lee, Sharlene; Niculescu-Duvaz, Dan; Good, Valerie M; Project, Cancer Genome; Jones, C. Michael et al. (2004-03-19). "Mechanism of Activation of the RAF-ERK Signaling Pathway by Oncogenic Mutations of B-RAF". Cell 116 (6): 855–867. doi:10.1016/S0092-8674(04)00215-6. ISSN 0092-8674. PMID 15035987.
- ↑ Weber, Arnim; Heinlein, Melanie; Dengjel, Jörn; Alber, Claudia; Singh, Prafull Kumar; Häcker, Georg (24 Mar 2016). "The deubiquitinase Usp27x stabilizes the BH3‐only protein Bim and enhances apoptosis". EMBO Reports 17 (5): 724–738. doi:10.15252/embr.201541392. ISSN 1469-221X. PMID 27013495.
- ↑ 19.0 19.1 Hu, H; Haas, S A; Chelly, J; Van Esch, H; Raynaud, M; de Brouwer, A P M; Weinert, S; Froyen, G et al. (2016). "X-exome sequencing of 405 unresolved families identifies seven novel intellectual disability genes". Molecular Psychiatry 21 (1): 133–148. doi:10.1038/mp.2014.193. ISSN 1359-4184. PMID 25644381.
- ↑ "genetics home reference". https://ghr.nlm.nih.gov/gene/KIF7.
 |

