Chemistry:α-Santalol
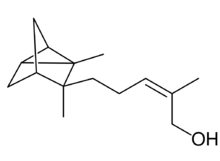
| |
| Names | |
|---|---|
| IUPAC name
(Z)-5-(2,3-Dimethyltricyclol[2.2.1.02,6]hept-3-yl)-2-methylpent-2-en-1-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H24O | |
| Molar mass | 220.356 g·mol−1 |
| Appearance | Liquid |
| Density | 0.9770 g/cm3 |
| Boiling point | 166 °C (331 °F; 439 K) |
| Practically insoluble | |
| Solubility in ethanol | Soluble |
| Solubility in diethyl ether | Soluble |
Chiral rotation ([α]D)
|
+10.3° |
Refractive index (nD)
|
1.5017 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317 | |
| P261, P272, P280, P302+352, P321, P333+313, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Related terpenes
|
β-Santalol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
α-Santalol, also referred to as alpha-santalol,[1] is an organic compound that is classified as a sesquiterpene. It comprises about 55% of the oil of sandalwood, another less abundant component being β-santalol. As of 2002, about 60 tons of sandalwood oil are produced annually by steam distillation of the heartwood of Santalum album. It is a valued component for perfumes.[2]
Because of concerns about the sustainability of sandalwood tree cultivation, scientists have developed routes to α-santalol and β-santalol via fermentation, including using Rhodobacter sphaeroides. BASF launched its version, Isiobionic Santalol, in July 2020.[3]
The oil content varies greatly within the different sandalwood species. This level is typically highest in S. album, S. paniculatum and S. yasi. The scent profile also changes considerably between the different species' oils.
References
- ↑ Bommareddy, AExpression error: Unrecognized word "et". (2019). "Medicinal properties of alpha-santalol, a naturally occurring constituent of sandalwood oil: review.". Nat. Prod. Res. 33 (4): 527–543. doi:10.1080/14786419.2017.1399387. PMID 29130352.
- ↑ Karl-Georg Fahlbusch; Franz-Josef Hammerschmidt; Johannes Panten; Wilhelm Pickenhagen; Dietmar Schatkowski; Kurt Bauer; Dorothea Garbe; Horst Surburg (2002). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_141. ISBN 3527306730.
- ↑ Bettenhausen, Craig (21 Nov 2020). "Making sandalwood oil without sandalwood trees". Chemical & Engineering News. https://cen.acs.org/business/5-new-technologies-making-impact/98/i46#Case-study-4-Making-sandalwood-oil-without-sandalwood-trees.
ru:Санталол
 |


