Chemistry:β-Santalol
From HandWiki
Short description: Chemical compound
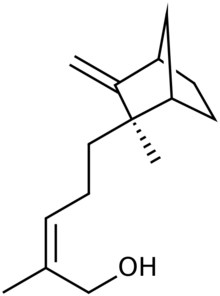
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2Z)-2-Methyl-5-[(1S,2R,4R)-2-methyl-3-methylidenebicyclo[2.2.1]heptan-2-yl]pent-2-en-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C15H24O | |
| Molar mass | 220.356 g·mol−1 |
| Appearance | Liquid |
| Density | 0.9717 g/cm3 |
| Boiling point | 177 °C (351 °F; 450 K) |
| Practically insoluble | |
| Solubility in ethanol | Soluble |
| Solubility in diethyl ether | Soluble |
Chiral rotation ([α]D)
|
−87.1° |
Refractive index (nD)
|
1.5100 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317 | |
| P261, P272, P280, P302+352, P321, P333+313, P363, P501 | |
| Related compounds | |
Related terpenes
|
α-Santalol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
β-Santalol is an organic compound that is classified as a sesquiterpene. It comprises about 20% of the oil of sandalwood, the major component being α-santalol. In 2002, about 60 tons of sandalwood oil were produced by steam distillation of the heartwood of Santalum album.[1]
Because of concerns about the sustainability of sandalwood tree cultivation, scientists have developed routes to α-santalol and β-santalol via fermentation, including using Rhodobacter sphaeroides. BASF launched its version, Isiobionic Santalol, in July 2020.[2]
References
- ↑ Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim: 2002. Published online: 15 January 2003; doi:10.1002/14356007.a11_141.
- ↑ Bettenhausen, Craig (November 11, 2021). "Making sandalwood oil without sandalwood trees". Chemical & Engineering News. https://cen.acs.org/business/5-new-technologies-making-impact/98/i46#Case-study-4-Making-sandalwood-oil-without-sandalwood-trees.
 |

