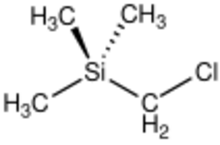
Chemistry:(Trimethylsilyl)methyl chloride
From HandWiki
| |
| Names | |
|---|---|
| Other names
(Chloromethyl)trimethylsilane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4H11ClSi | |
| Molar mass | 122.67 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.886 g cm−3 |
| Boiling point | 97–98 °C (207–208 °F; 370–371 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H225, H315, H319, H335, H411 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P273, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P370+378, P391, P403+233, P403+235, P405 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
(Trimethylsilyl)methyl chloride is the organosilicon compound with the formula (CH3)3SiCH2Cl. A colorless, volatile liquid, it is an alkylating agent that is employed in organic synthesis, especially as a precursor to (trimethylsilyl)methyllithium. In the presence of triphenylphosphine, it olefinates benzophenones:[2]
- (CH
3)
3SiCH
2Cl + PPh
3 + Ar
2C=O → Ar
2C=CH
2 + OPPh
3 + (CH
3)
3SiCl
See also
- Trimethylsilyl chloride, a silyl chloride
References
- ↑ "Chloromethyltrimethylsilane" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/75361#section=Safety-and-Hazards.
- ↑ Hamann, Lawrence G.; Jones, Todd K. (2001). "(Chloromethyl)trimethylsilane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc129. ISBN 0471936235.
 |

