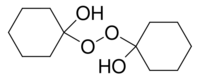
Chemistry:1,1'-Dihydroxydicyclohexyl peroxide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
1-(1-hydroxycyclohexyl)peroxycyclohexan-1-ol
| |
| Other names
Bis(1-hydroxycyclohexyl) peroxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3106 |
| |
| |
| Properties | |
| C12H22O4 | |
| Molar mass | 230.304 g·mol−1 |
| Appearance | white solid |
| Melting point | 66–68 °C (151–154 °F; 339–341 K) |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H240, H302, H314 | |
| P210, P220, P234, P260, P264, P270, P280, P301+312, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P330, P363, P370+378, P370+380+375, P403+235, P405, P411, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1,1′-Dihydroxydicyclohexyl peroxide is an organic compound with the formula (C6H10OH)2O2. It is one of the peroxides derived from the reaction of cyclohexanone and hydrogen peroxide. Upon treatment with acid and additional peroxide, it converts to the cyclic diperoxide, bis(cyclohexylidene peroxide), (C6H10)2(O2)2.[1][2]
1,1′-Dihydroxydicyclohexyl peroxide is a catalyst for radical-initiated vulcanization.
References
- ↑ Story, Paul R.; Lee, Bunge; Bishop, Clyde E.; Denson, Donald D.; Busch, Peter (1970). "Macrocyclic Synthesis. II. Cyclohexanone Peroxides". The Journal of Organic Chemistry 35 (9): 3059–3062. doi:10.1021/jo00834a042.
- ↑ McCullough, Kevin J.; Morgan, Alistair R.; Nonhebel, Derek C.; Pauson, Peter L.; White, Graham J. (1980). "Ketone-Derived Peroxides. Part 1. Synthetic Methods". Journal of Chemical Research, Synopses: 34.
 |

