Chemistry:1,1,1-Tris(diphenylphosphinomethyl)ethane
| |
| Names | |
|---|---|
| Preferred IUPAC name
{2-[(Diphenylphosphanyl)methyl]-2-methylpropane-1,3-diyl}bis(diphenylphosphane) | |
| Other names
Triphos; tdppme; tdme
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C41H39P3 | |
| Molar mass | 624.67 g/mol |
| Appearance | white crystals |
| Melting point | 99 to 102 °C (210 to 216 °F; 372 to 375 K) |
| Insoluble | |
| Hazards | |
| Safety data sheet | Triphos MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
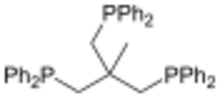
1,1,1-Tris(diphenylphosphinomethyl)ethane, also called Triphos, is an organophosphorus compound with the formula CH3C[CH2PPh2]3. An air-sensitive white solid, it is a tripodal ligand ("three-legged") of idealized C3v symmetry. It was originally prepared by the reaction of sodium diphenylphosphide and CH3C(CH2Cl)3:[1]
- 3 Ph2PNa + CH3C(CH2Cl)3 → CH3C[CH2PPh2]3 + 3 NaCl
It forms complexes with many transition metals, usually as a tripodal ligand.[2] Such complexes are used to analyze mechanistic aspects of homogeneous catalysts.[3] For example, rhodium forms complexes with CH3C[CH2PPh2]3 like [(triphos)RhCl(C2H4)], [(triphos)RhH(C2H4)], and [(triphos)Rh(C2H5)(C2H4)], provide model intermediates in the catalytic cycle for hydrogenation of alkenes.[4]
Triphos sometimes behaves as a bidentate ligand. Illustrative cases include fac-[Mn(CO)3Br(η2-triphos)] and [M(CO)4(η2-triphos)], where M is Cr, Mo, or W. Triphos serves as a tridentate-bridging ligand in an icosahedral Au13 cluster. The phosphine bridges three chlorogold(I) groups to form the tripod molecule of trichloro-1,1,1-(diphenylphosphinomethyl)ethanetrigold(I), CH3C[CH2PPh2AuCl]3.[5]
Related ligands
- Tris(aminomethyl)ethane, a tripodal triamine (CH3C(CH2NH2)3)
- Bis(diphenylphosphinoethyl)phenylphosphine (PhP(C2H4PPh2)2)
References
- ↑ W. Hewertson; H. R. Watson (1962). "283. The preparation of di- and tri-tertiary phosphines". J. Chem. Soc.: 1490–1494. doi:10.1039/JR9620001490.
- ↑ Huttner, G.; Strittmatter, J.; Sandhoefner, S. (2004). Comprehensive Coordination Chemistry II. 1. pp. 297–322. doi:10.1016/B0-08-043748-6/01082-3. ISBN 9780080437484.
- ↑ Bianchini, Claudio; Marchi, Andrea; Marvelli, Lorenza; Peruzzini, Maurizio; Romerosa, Antonio; Rossi, Roberto (1996). "Multiple Re-C Bonds at the [{MeC(CH2PPh2)3}Re(CO)2]+ Auxiliary". Organometallics 15: 3804. doi:10.1021/om9602264.
- ↑ Bianchini, Claudio; Meli, Andrea; Peruzzini, Maurizio; Vizza, Francesco (1990). "Tripodal Polyphosphine Ligands in Homogeneous Catalysis. 1. Hydrogenation and Hydroformylation of Alkynes and Alkenes Assisted by Organorhodium Complexes with MeC(CH2PPh2)3". Organometallics 9: 226–240. doi:10.1021/om00115a035.
- ↑ Cooper, Mervyn K.; Henrick, Kim; McPartlin, Mary; Latten, Jozef L. (1982). "The synthesis and X-ray structure of trichloro-1,1,1-(diphenylphosphinomethyl)ethanetrigold(I)". Inorganica Chimica Acta 65 (2): L185. doi:10.1016/S0020-1693(00)93540-0.
 |

