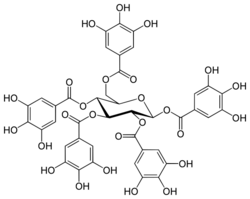
Chemistry:1,2,3,4,6-Pentagalloyl glucose
| |
| Names | |
|---|---|
| IUPAC name
β-D-Glucopyranose pentakis(3,4,5-trihydroxybenzoate)
| |
| Systematic IUPAC name
(2S,3R,4S,5R,6R)-6-{[(3,4,5-Trihydroxybenzoyl)oxy]methyl}oxane-2,3,4,5-tetrayl tetrakis(3,4,5-trihydroxybenzoate) | |
| Other names
1,2,3,4,6-Penta-O-galloyl-β-D-glucose
1,2,3,4,6-Pentakis-O-galloyl-beta-D-glucose beta-Penta-O-galloyl-glucose PGG 1,2,3,4,6-Penta-O-galloyl-beta-D-glucose | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C41H32O26 | |
| Molar mass | 940.681 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,2,3,4,6-Pentagalloylglucose is the pentagallic acid ester of glucose. It is a gallotannin and the precursor of ellagitannins.[1]
Pentagalloyl glucose can precipitate proteins,[2] including human salivary α-amylase.[3]
Natural occurrence
1,2,3,4,6-Pentagalloyl glucose can be found in Punica granatum (pomegranate),[4] Elaeocarpus sylvestris,[5] Rhus typhina (Staghorn sumac),[6] Paeonia suffruticosa (Tree Peony),.,[7] Mangifera indica (mango) [8] and Bouea macrophylla Griffith (maprang).[9]
Biosynthesis
The enzyme beta-glucogallin-tetrakisgalloylglucose O-galloyltransferase uses 1-O-galloyl-beta-D-glucose and 1,2,3,6-tetrakis-O-galloyl-beta-D-glucose to produce D-glucose and pentagalloyl glucose.
Metabolism
Tellimagrandin II is formed from pentagalloyl glucose by oxidative dehydrogenation and coupling of 2 galloyl groups.
β-glucogallin: 1,2,3,4,6-pentagalloyl-β-d-glucose galloyltransferase is an enzyme found in the leaves of Rhus typhina that catalyzes the galloylation of 1,2,3,4,6-penta-O-galloyl-β-D-glucose to 3-O-digalloyl-1,2,4,6-tetra-O-galloyl-β-d-glucose (hexa-galloylglucose).[6]
Chemistry
Pentagalloyl glucose can undergo oxidation reactions which are depending on the pH.[10]
Research
Pentagalloyl glucose has been studied for its potential use as an antimicrobial, anti-inflammatory, anticarcinogenic, antidiabetic, and antioxidant.[11] It has also been studied for radioprotection.[5] This compound helps stabilize the elastin and collagen in vascular tissues[12] and restores the biomechanical properties of arterial ECM.[13] In addition, pentagalloyl glucose has shown to reduce arterial calcification and helps promote extracellular matrix preservation in animal models of abdominal aortic aneurysm.[14] In vitro studies with mouse C2C12 myoblast cells have shown the PGG helps in lowering reactive oxygen species (ROS) and matrix metalloproteinase-2 (MMP-2) expression in a dose-dependent manner. [15]
References
- ↑ "Biosynthesis of gallotannins: beta-glucogallin-dependent formation of 1,2,3,4,6-pentagalloylglucose by enzymatic galloylation of 1,2,3,6-tetragalloylglucose". Archives of Biochemistry and Biophysics 273 (1): 58–63. August 1989. doi:10.1016/0003-9861(89)90161-6. PMID 2757399.
- ↑ "Mechanisms of Protein Precipitation for Two Tannins, Pentagalloyl Glucose and Epicatechin16(4→8) Catechin (Procyanidin)". Journal of Agricultural and Food Chemistry 46 (7): 2590–2595. 1998. doi:10.1021/jf971097k.
- ↑ "Evidence for pentagalloyl glucose binding to human salivary alpha-amylase through aromatic amino acid residues". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics 1794 (2): 291–6. February 2009. doi:10.1016/j.bbapap.2008.10.012. PMID 19038368.
- ↑ Tanaka, Takashi; Nonaka, Gen-Ichiro; Nishioka, Itsuo (1985). "Punicafolin, an ellagitannin from the leaves of Punica granatum". Phytochemistry 24 (9): 2075. doi:10.1016/S0031-9422(00)83125-8.
- ↑ 5.0 5.1 "Elaeocarpus sylvestris modulates gamma-ray-induced immunosuppression in mice: implications in radioprotection". Phytotherapy Research 22 (8): 1046–51. August 2008. doi:10.1002/ptr.2430. PMID 18570220.
- ↑ 6.0 6.1 Niemetz, Ruth; Gross, Georg G (1998). "Gallotannin biosynthesis: Purification of β-glucogallin: 1,2,3,4,6-pentagalloyl-β-d-glucose galloyltransferase from sumac leavesfn1fn1In honour of Professor G. H. Neil Towers' 75th birthday". Phytochemistry 49 (2): 327. doi:10.1016/S0031-9422(98)00014-4.
- ↑ "A traditional medicinal herb Paeonia suffruticosa and its active constituent 1,2,3,4,6-penta-O-galloyl-beta-D-glucopyranose have potent anti-aggregation effects on Alzheimer's amyloid beta proteins in vitro and in vivo". Journal of Neurochemistry 109 (6): 1648–57. June 2009. doi:10.1111/j.1471-4159.2009.06069.x. PMID 19457098.
- ↑ Torres-León, Cristian; Rojas, Romeo; Aguilar, Cristóbal (2017). "Extraction of antioxidants from mango seedkernel: Optimization assisted by microwave". Food and Bioproducts Processing 105: 188–196. doi:10.1016/j.fbp.2017.07.005.
- ↑ Kantapan, Jiraporn; Paksee, Siwaphon; Chawapun, Pornthip; Sangthong, Padchanee; Dechsupa, Nathupakorn (2020). "Pentagalloyl Glucose- and Ethyl Gallate-Rich Extract from Maprang Seeds Induce Apoptosis in MCF-7 Breast Cancer Cells through Mitochondria-Mediated Pathway". Evidence-Based Complementary and Alternative Medicine 2020: 1–19. doi:10.1155/2020/5686029. PMID 32382295.
- ↑ "Reaction pH and protein affect the oxidation products of beta-pentagalloyl glucose". Free Radical Research 39 (2): 117–24. February 2005. doi:10.1080/10715760400013789. PMID 15763959.
- ↑ "Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties". Journal of Functional Foods 37: 176–189. 2017. doi:10.1016/j.jff.2017.07.045.
- ↑ "Pentagalloyl Glucose and Its Functional Role in Vascular Health: Biomechanics and Drug-Delivery Characteristics". Annals of Biomedical Engineering 47 (1): 39–59. January 2019. doi:10.1007/s10439-018-02145-5. PMID 30298373.
- ↑ Patnaik, Sourav S.; Piskin, Senol; Pillalamarri, Narasimha Rao; Romero, Gabriela; Escobar, G. Patricia; Sprague, Eugene; Finol, Ender A. (2019-07-03). "Biomechanical Restoration Potential of Pentagalloyl Glucose after Arterial Extracellular Matrix Degeneration". Bioengineering 6 (3): 58. doi:10.3390/bioengineering6030058. ISSN 2306-5354. PMID 31277241.
- ↑ Anderson, Jennifer L.; Niedert, Elizabeth E.; Patnaik, Sourav S.; Tang, Renxiang; Holloway, Riley L.; Osteguin, Vangelina; Finol, Ender A.; Goergen, Craig J. (January 2021). "Animal Model Dependent Response to Pentagalloyl Glucose in Murine Abdominal Aortic Injury" (in en). Journal of Clinical Medicine 10 (2): 219. doi:10.3390/jcm10020219. PMID 33435461.
- ↑ Arnold, Frances; Muzzio, Nicolas; Patnaik, Sourav S.; Finol, Ender A.; Romero, Gabriela (2021-05-24). "Pentagalloyl Glucose-Laden Poly(lactide-co-glycolide) Nanoparticles for the Biomechanical Extracellular Matrix Stabilization of an In Vitro Abdominal Aortic Aneurysm Model". ACS Applied Materials & Interfaces 13 (22): 25771–25782. doi:10.1021/acsami.1c05344. ISSN 1944-8252. PMID 34030437. https://pubmed.ncbi.nlm.nih.gov/34030437.
 |

