Chemistry:1,2,3-Cyclohexatriene
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Cyclohexa-1,2,3-triene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| |
| |
| Properties | |
| C6H6 | |
| Molar mass | 78.114 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1,2,3-Cyclohexatriene is an unstable chemical compound with the molecular formula C
6H
6.[1] It is an unusual isomer of benzene in which the three double bonds are cumulated.
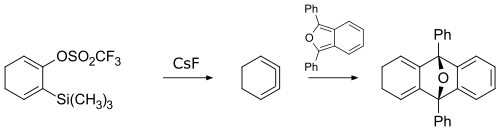
In 1990, 1,2,3-cyclohexatriene was first prepared by reacting a cyclohexadiene derivative with cesium fluoride.[2] The product was too reactive to be isolated on its own, so its existence was confirmed by trapping via a cycloaddition reaction.
1,2,3-Cyclohexatriene and its derivatives undergo a variety of reactions including cycloadditions, nucleophilic additions, and σ-bond insertions,[3] and therefore they can be versatile reagents for organic synthesis.[4]
References
- ↑ "Benzene's forgotten isomer takes centre stage in organic synthesis". May 2, 2023. https://www.chemistryworld.com/news/benzenes-forgotten-isomer-takes-centre-stage-in-organic-synthesis/4017364.article.
- ↑ Shakespeare , William C. ; Johnson , Richard P. (1990 ). "1,2,3-cyclohexatriene and cyclohexen-3-yne: Two new highly strained C6H6 isomers ". Journal of the American Chemical Society 112 (23 ): 8578–8579 . doi:10.1021/ja00179a050 .
- ↑ Kelleghan, Andrew V.; Bulger, Ana S.; Witkowski, Dominick C.; Garg, Neil K. (2023). "Strain-promoted reactions of 1,2,3-cyclohexatriene and its derivatives". Nature 618 (7966): 748–754. doi:10.1038/s41586-023-06075-8. PMID 37075803.
- ↑ "A fresh look at 1,2,3-cyclohexatriene shows it could be used as a versatile reagent in organic synthesis". May 3, 2023. https://phys.org/news/2023-05-fresh-cyclohexatriene-versatile-reagent-synthesis.html.
 |