Chemistry:1,8-Naphthalic anhydride
From HandWiki
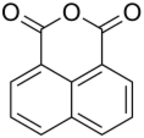
| |
| Names | |
|---|---|
| Preferred IUPAC name
1H,3H-Naphtho[1,8-cd]pyran-1,3-dione | |
| Other names
1,8-Naphthalenedicarboxylic acid anhydride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H6O3 | |
| Molar mass | 198.177 g·mol−1 |
| Appearance | white solid |
| Melting point | 269–276 °C (516–529 °F; 542–549 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H317, H319, H335 | |
| P261, P264, P271, P272, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P333+313, P337+313, P362, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1,8-Naphthalic anhydride is an organic compound with the formula C10H6(C2O3). It is one of three isomers of naphthalic anhydride, the other two being the 1,2- and the 2,3-derivatives. The 1,8-isomer is prepared by aerobic oxidation of acenaphthene.[1] 2,6-naphthalenedicarboxylic acid can be prepared from this anhydride.[2] 1,8-Naphthalic anhydride is a precursor to the 4-chloro and 4,5-dichloro derivatives. These chloride groups are susceptible to displacement by amines and alkoxides,[3] giving rise, ultimately, to a large family of naphthalimides, which are used as optical brighteners.[4]
Derivatives include: Alrestatin,...
References
- ↑ Griesbaum, Karl; Behr, Arno; Biedenkapp, Dieter; Voges, Heinz-Werner; Garbe, Dorothea; Paetz, Christian; Collin, Gerd; Mayer, Dieter et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_227.
- ↑ Raecke, Bernhard; Schirp, Hubert (1960). "2,6-Naphthalenedicarboxylic acid". Org. Synth. 40: 71. doi:10.15227/orgsyn.040.0071.
- ↑ Tyman, John; Ghorbanian, Shoreh; Muir, M.; Tychopoulous, Vasiliki; Bruce, Ian; Fisher, Ian (1989). "Improved Nucleophilic Displacements in N-Methyl Pyrrolidinone as a Solvent". Synthetic Communications 19 (1–2): 179–188. doi:10.1080/00397918908050968.
- ↑ Haremsa, Sylke (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_059.
 |

