Chemistry:1-Ethyl-3-methylimidazolium chloride
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Ethyl-1-methyl-3H-imidazol-1-ium chloride | |
| Other names
[EMIM]Cl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H11ClN2 | |
| Molar mass | 146.62 g·mol−1 |
| Melting point | 77 to 79 °C (171 to 174 °F; 350 to 352 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302, H315, H319 | |
| P264, P270, P280, P301+312, P302+352, P305+351+338, P321, P330, P332+313, P337+313, P362, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
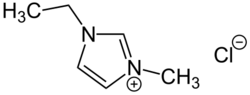
1-Ethyl-3-methylimidazolium chloride or [EMIM]Cl is an ionic liquid that can be used in cellulose processing.[1][2] The cation consists of a five-membered ring with two nitrogen and three carbon atoms, i.e. a derivative of imidazole, with ethyl and methyl groups substituted at the two nitrogen atoms.[3] Its melting point is 77–79 °C.[4]
References
- ↑ Scientists Propose a More Efficient Way to Make Ethanol, The New York Times, March 2, 2010
- ↑ Joseph B. Binder and Ronald T. Raines (2010). "Fermentable sugars by chemical hydrolysis of biomass". PNAS 107 (10): 4516–4521. doi:10.1073/pnas.0912073107. PMID 20194793. PMC 2842027. http://www.pnas.org/content/early/2010/03/02/0912073107.full.pdf.
- ↑ 1-Ethyl-3-methylimidazolium chloride, chemexper.com
- ↑ MSDS
 |

