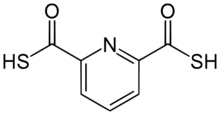
Chemistry:2,6-Pyridinedicarbothioic acid
| |
| |
| Names | |
|---|---|
| IUPAC name
2,6-Pyridinedicarbothioic acid
| |
| Preferred IUPAC name
Pyridine-2,6-bis(carbothioic S-acid) | |
| Other names
PDTC, dithiopyridinedicarbothioic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H5O2S2 | |
| Molar mass | 185.24 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.415 g/cm3 |
| Melting point | 97 to 99 °C (207 to 210 °F; 370 to 372 K) |
| Boiling point | 404.4 °C (759.9 °F; 677.5 K) |
| 1000 g/L (5.02 mol/L) | |
| Hazards | |
| Main hazards | acidic |
| Flash point | 198.4 °C (389.1 °F; 471.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,6-Pyridinedicarbothioic acid (PDTC) is an organosulfur compound that is produced by some bacteria. It functions as a , a low molecular weight compound that scavenges iron. Siderophores solubilize compounds by forming strong complexes. PDTC is secreted by the soil bacteria Pseudomonas stutzeri and Pseudomonas putida.[1]
Synthesis and biosynthesis
PDTC can be synthesized in the laboratory by treating the diacid dichloride of pyridine-2,6-dicarboxylic with H2S in pyridine:
- NC
5H
3(COCl)
2 + 2 H
2S + 2 C
5H
5N → [C
5H
5NH+
][HNC
5H
3(COS)−
2] + [C
5H
5NH]Cl
This route produces the pyridinium salt of pyridinium-2,6-dicarbothioate. Treatment of this orange-colored salt with sulfuric acid gives colorless PDTC, which can then be extracted with dichloromethane.[2]
The biosynthesis of PDTC remains unclear although some insights can be deduced from the genetics.[3] It is suggested that Pseudomonas stutzeri may have acquired at least one of the genes by lateral transfer from mycobacteria.[4] In a proposed biosynthetic sequence pyridine-2,6-dicarboxylic acid, a known bacterial metabolite,[4] is activated as its bis-adenosine monophosphate (AMP) derivative. The sulfur donor and its activation remain uncertain.[5]
Coordination chemistry
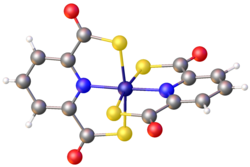
PDTC binds to both Fe2+ and Fe3+. The ferric complex is brown, whereas the ferrous complex is blue. In the presence of air, the ferrous complex oxidizes to the ferric compound.[7] It is iron selective[4] as only the Fe complex is soluble in water. PDTC is produced mainly during the exponential phase of bacterial growth. The conditions at which Pseudomonas produces PDTC is 25 °C, pH=8 and sufficient aeration.[5]
See also
References
- ↑ Budzikiewicz, Herbert (2010). "Microbial Siderophores". in Kinghorn, A. Douglas. Fortschritte der Chemie organischer Naturstoffe / Progress in the Chemistry of Organic Natural Products, Vol. 92. 92. pp. 1–75. doi:10.1007/978-3-211-99661-4_1. ISBN 978-3-211-99660-7.
- ↑ Hildebrand, U.; Ockels, W.; Lex, J.; Budzikiewicz, H. (1983). "Zur Struktur Eines 1:1-Adduktes von Pyridin-2,6-Dicarbothiosäure und Pyridin". Phosphorus and Sulfur and the Related Elements 16 (3): 361–364. doi:10.1080/03086648308080490.
- ↑ Cortese, Marc S; Caplan, Allan B; Crawford, Ronald L (2002). "Structural, functional, and evolutionary analysis of moeZ, a gene encoding an enzyme required for the synthesis of the Pseudomonas metabolite, pyridine-2,6-bis(thiocarboxylic acid)". BMC Evolutionary Biology 2: 8. doi:10.1186/1471-2148-2-8. PMID 11972321.
- ↑ 4.0 4.1 4.2 Cortese, Marc S.; Paszczynski, Andrzej; Lewis, Thomas A.; Sebat, Jonathan L.; Borek, Vladimir; Crawford, Ronald L. (2002). "Metal chelating properties of pyridine-2,6-bis(thiocarboxylic acid) produced by Pseudomonas spp. And the biological activities of the formed complexes". BioMetals 15 (2): 103–120. doi:10.1023/A:1015241925322. PMID 12046919.
- ↑ 5.0 5.1 Budzikiewicz, H. (2003). "Heteroaromatic monothiocarboxylic acids from Pseudomonas spp". Biodegradation 14 (2): 65–72. doi:10.1023/A:1024012015127. PMID 12877462.
- ↑ Hildebrand, U.; Lex, J.; Taraz, K.; Winkler, S.; Ockels, W.; Budzikiewicz, H. (1984). "Untersuchungen zum Redox-System Bis-(pyridin-2,6-dicarbothioato)-Ferrat(II) /-Ferrat(III) [1]". Zeitschrift für Naturforschung B 39 (11): 1607–1613. doi:10.1515/znb-1984-1123.
- ↑ Ockels, W., Roemer, A., Budzikiewicz, H., Korth, H., Pulverer, G., "Bacterial constituents. II. An iron(II) complex of pyridine-2,6-di-(monothiocarboxylic acid) - a novel bacterial metabolic product", Tetrahedron Lett. 1978, 3341. doi:10.1016/S0040-4039(01)85634-3
 |
