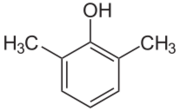
Chemistry:2,6-Xylenol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Dimethylphenol | |
| Other names
2-Hydroxy-m-xylene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.167 g·mol−1 |
| Appearance | white solid |
| Density | 1.132 g/cm3 |
| Melting point | 43 to 45 °C (109 to 113 °F; 316 to 318 K) |
| Boiling point | 203 °C (397 °F; 476 K) |
| Hazards | |
| Flash point | 86 °C (187 °F; 359 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,6-Xylenol is a chemical compound which is one of the six isomers of xylenol. It is also commonly known as 2,6-dimethylphenol (DMP). It is a colorless solid.
Production
2,6-DMP is produced by the methylation of phenol. With production >100,000 tons/y, it is the most important xylenol. The methylation is carried out by contacting gaseous phenol and methanol at elevated temperatures in the presence of a solid acid catalyst:[2][3]
- C
6H
5OH + 2 CH
3OH → (CH
3)
2C
6H
3OH + 2 H
2O
Challenges associated with the production is the similarity of the boiling points of cresols and this xylenol.
Reactions
2,6-Xylenol is susceptible to oxidative coupling leading to polymers and dimers.[4]
Acid-catalyzed condensation of 2,6-xylenol gives tetramethylbisphenol A. An analogue of bisphenol A, this bisphenol is used in the production of some polycarbonates. 2,6-Xylenol reacts with ammonia to give 2,6-dimethylaniline.[2]
Toxicity
Its LD50 (oral, rats) ranges from 296-1750 mg/kg.[2]
References
- ↑ 2,6-Xylenol at Sigma-Aldrich
- ↑ 2.0 2.1 2.2 Fiege, Helmut (2000). "Cresols and Xylenols". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a08_025. ISBN 3527306730.
- ↑ Zukowski, W.; Berkowicz, G.; Baron, J.; Kandefer, S.; Jamanek, D.; Szarlik, S.; Wielgosz, Z.; Zielecka, M. (2014). "Selective phenol methylation to 2,6-dimethylphenol in a fluidized bed of iron-chromium mixed oxide catalyst with o-cresol circulation". Chemistry Central Journal 8 (1): 51. doi:10.1186/s13065-014-0051-6. PMID 25342964. PMC 4172955. https://core.ac.uk/download/pdf/81194098.pdf.
- ↑ Selective oxidative para C–C dimerization of 2,6-dimethylphenol Christophe Boldron, Guillem Aromí, Ger Challa, Patrick Gamez and Jan Reedijk Chemical Communications, 2005, (46), 5808 - 5810 Abstract
 |

