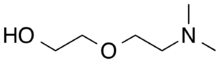
Chemistry:2-(2-(Dimethylamino)ethoxy)ethanol
| |
| Names | |
|---|---|
| IUPAC name
2-[2-(dimethylamino)ethoxy]ethanol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H15NO2 | |
| Molar mass | 133.191 g·mol−1 |
| Boiling point | 95 °C (203 °F; 368 K) |
Refractive index (nD)
|
1.442 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Danger |
| H312, H314 | |
| P260, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P280, P301+330+331, P302+352, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P321, P362+364Script error: No such module "Preview warning".Category:GHS errors, P363, P405, P501 | |
| Flash point | 93 °C (199 °F; 366 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-[2-(Dimethylamino)ethoxy]ethanol is an organic compound with the molecular formula C6H15NO2 and is a liquid at room temperature. Dimethylaminoethoxyethanol is polyfunctional, having a tertiary amine, ether and hydroxyl functionality. Like other organic amines, it acts as a weak base.
Manufacture
Dimethylaminoethoxyethanol is manufactured by reacting dimethylamine and ethylene oxide.[2] Other methods are also available producing streams rich in the substance which then need to be further purified.[3]
Uses
As dimethylaminoethoxyethanol is weakly basic,[4] it has been studied as a method of absorbing Greenhouse gases and in particular carbon dioxide.[5][6][7][8]
Dimethylaminoethoxyethanol is used extensively in surfactants which have also been evaluated as corrosion inhibitors.[9] Surfactants prepared are usually cationic and may also be used as a biocide.[10] This is particularly important for oilfield applications against Sulfate-reducing microorganisms.
The material has other uses which include:
- General such as clays, intermediates, plasticizers and adhesives.[11]
- As a catalyst and especially for polyurethanes.[12][13]
- Process regulators
- Propellants and blowing agents
Toxicity
The toxicity of dimethylaminoethoxyethanol has been extensively studied.[14]
References
- ↑ "2-[2-(Dimethylamino)ethoxyethanol"] (in en). https://pubchem.ncbi.nlm.nih.gov/compound/74348#section=Safety-and-Hazards.
- ↑ Frank, H., 2007. Preparation of N, N-Dimethylaminoethoxyethanol by Reacting Reacting Di-methylamine with Ethylene Oxide US Patent
- ↑ US8907084B2 - Process for the preparation of 2-(2-aminoethoxy) ethanol (2AEE) and morpholine with 2AEE: morpholine >3 - Google Patents
- ↑ Liu, Gao; Nguyen, William (Hoang Chi Hieu); Henni, Amr (March 2023). "Experimental determination of dissociation constants (p K a ) for N -(2-aminoethyl)-1,3-propanediamine, 2-methylpentamethylene diamine, N,N -dimethyldipropylenetriamine, 3,3′- diamino-N -methyldipropyl-amine, Bis [2-( N,N -dimethylamino)ethyl]ether, 2-[2-(dimethyl-amino) ethoxy] ethanol, 2-(dibutylamino) ethanol, and N -propylethanol-amine and modeling with artificial neural network" (in en). AIChE Journal 69 (3). doi:10.1002/aic.17923. ISSN 0001-1541.
- ↑ Delavari, Mohammad; Khajenoori, Maryam; Zoghi, Ali T. (2023-11-01). "Equilibrium absorption of CO2 in aqueous solution of N-dimethylamino ethanol and 2-(ethylamino)ethanol, measuring and thermodynamic modeling". The Journal of Chemical Thermodynamics 186: 107142. doi:10.1016/j.jct.2023.107142. ISSN 0021-9614. https://www.sciencedirect.com/science/article/pii/S0021961423001398.
- ↑ Du, Yang; Yuan, Ye; Wang, Yukai; Rochelle, Gary T. (2017-07-01). "Thermally Degraded Diglycolamine/Dimethylaminoethoxyethanol for CO2 Capture". Energy Procedia. 13th International Conference on Greenhouse Gas Control Technologies, GHGT-13, 14-18 November 2016, Lausanne, Switzerland 114: 1737–1750. doi:10.1016/j.egypro.2017.03.1912. ISSN 1876-6102.
- ↑ Zhong, Xingyang; Li, Chao'en; Hu, Xiayi; Zhang, Rui (2023-10-15). "A modified semi-empirical model for correlating and predicting CO2 equilibrium solubility in aqueous 2-[2-(dimethylamino)ethoxyethanol solution"]. Separation and Purification Technology 323: 124364. doi:10.1016/j.seppur.2023.124364. ISSN 1383-5866. https://www.sciencedirect.com/science/article/pii/S1383586623012728.
- ↑ Rodier, Laurence; Ballerat-Busserolles, Karine; Coxam, Jean-Yves (2010-06-01). "Enthalpy of absorption and limit of solubility of CO2 in aqueous solutions of 2-amino-2-hydroxymethyl-1,3-propanediol, 2-[2-(dimethyl-amino)ethoxy ethanol, and 3-dimethyl-amino-1-propanol at T=(313.15 and 353.15)K and pressures up to 2MPa"]. The Journal of Chemical Thermodynamics 42 (6): 773–780. doi:10.1016/j.jct.2010.01.015. ISSN 0021-9614. https://www.sciencedirect.com/science/article/pii/S0021961410000285.
- ↑ Fouda, A. S.; Rashwan, S. M.; Shaban, Samy M.; Ibrahim, Hoyeda E.; Elbhrawy, M. F. (2018-09-01). "Evaluation of a novel cationic surfactant based on 2-(2 (dimethylamino)ethoxy)ethanol as a corrosion inhibitor for carbon steel 1018 in 1.0MHCl solution". Egyptian Journal of Petroleum 27 (3): 295–306. doi:10.1016/j.ejpe.2017.05.001. ISSN 1110-0621.
- ↑ Shaban, Samy M.; Fouda, A. S.; Rashwan, S. M.; Ibrahim, Hoyeda E.; El-Bhrawy, M. F. (2016-09-01). "Synthesis and characterization of newly cationic surfactants based on 2-(2-(dimethylamino)ethoxy)ethanol: physiochemical, thermodynamic and evaluation as biocide". Journal of Molecular Liquids 221: 224–234. doi:10.1016/j.molliq.2016.05.088. ISSN 0167-7322. https://www.sciencedirect.com/science/article/pii/S0167732216304603.
- ↑ "2-(2-DIMETHYLAMINO ETHOXY)ETHANOL" (in tr). https://www.atamanchemicals.com/2--2-dimethylamino-ethoxy-ethanol_u26733/.
- ↑ "Lupragen N 107 - Dimethylaminoethoxyethanol" (in en-US). https://products.basf.com/global/en/ci/lupragen%C2%AE%20n%20107%20-%20dimethylaminoethoxyethanol-30036754.html.
- ↑ Espinosa, Kurt; Schmitz, Jonathan; Fitzhenry, J.J.; Beck, Madison; Watkins, Linette (May 2022). "Kinetic and stability study on the immobilized enzymatic step of one-pot dimerization of 2-[2-(dimethylamino)ethoxyethanol"] (in en). The FASEB Journal 36 (S1). doi:10.1096/fasebj.2022.36.S1.0R756. ISSN 0892-6638. https://faseb.onlinelibrary.wiley.com/doi/10.1096/fasebj.2022.36.S1.0R756.
- ↑ Leung, H. W; Frantz, S. W; Ballantyne, B (2002-07-01). "Toxicity and pharmacokinetics of 2-(2-dimethylaminoethoxy)ethanol following cutaneous dosing". Food and Chemical Toxicology 40 (7): 1033–1040. doi:10.1016/S0278-6915(02)00010-8. ISSN 0278-6915. PMID 12065226. https://www.sciencedirect.com/science/article/pii/S0278691502000108.
 |

