Chemistry:2-Aminoisobutyric acid
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Amino-2-methylpropanoic acid | |
| Other names
α-Aminoisobutyric acid
2-Methylalanine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H9NO2 | |
| Molar mass | 103.12 g/mol |
| Appearance | white crystalline powder |
| Density | 1.09 g/mL |
| Boiling point | 204.4 °C (399.9 °F; 477.5 K) |
| soluble | |
| Acidity (pKa) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
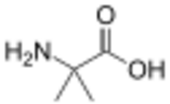
2-Aminoisobutyric acid (also known as α-aminoisobutyric acid, AIB, α-methylalanine, or 2-methylalanine) is the non-proteinogenic amino acid with the structural formula H2N-C(CH3)2-COOH. It is rare in nature, having been only found in meteorites,[1] and some antibiotics of fungal origin, such as alamethicin and some lantibiotics.
Synthesis
In the laboratory, 2-aminoisobutyric acid may be prepared from acetone cyanohydrin, by reaction with ammonia followed by hydrolysis.[2] Industrial scale synthesis can be achieved by the selective hydroamination of methacrylic acid.
Biological activity
2-Aminoisobutyric acid is not one of the proteinogenic amino acids and is rather rare in nature (cf. non-proteinogenic amino acids). It is a strong helix inducer in peptides due to Thorpe–Ingold effect of its gem-dimethyl group.[3] Oligomers of AIB form 310 helices.
Ribosomal incorporation into peptides
2-Aminoisobutyric acid is compatible with ribosomal elongation of peptide synthesis. Katoh et al. used flexizymes[4] and an engineered a tRNA body to enhance the affinity of aminoacylated AIB-tRNA species to elongation factor P.[5] The result was an increased incorporation of AIB into peptides in a cell free translation system. Iqbal et al.. used an alternative approach of creating an editing deficient valine—tRNA ligase to synthesize aminoacylated AIB-tRNAVal. The aminoacylated tRNA was subsequently used in a cell-free translation system to yield AIB-containing peptides.[6]
References
- ↑ "Immune System of Humans, Other Mammals Could Struggle to Fight Extraterrestrial Microorganisms". Science News. 23 July 2020. http://www.sci-news.com/space/mammalian-immune-system-extraterrestrial-microorganisms-08668.html.
- ↑ Clarke, H. T.; Bean, H. J. (1931). "α-Aminoisobutyric acid". Organic Syntheses 11: 4. http://www.orgsyn.org/demo.aspx?prep=cv2p0029.; Collective Volume, 2, pp. 29.
- ↑ Toniolo, C.; Crisma, M.; Formaggio, F.; Peggion, C. (2001). "Control of peptide conformation by the Thorpe-Ingold effect (C alpha-tetrasubstitution)". Biopolymers 60 (6): 396–419. doi:10.1002/1097-0282(2001)60:6<396::AID-BIP10184>3.0.CO;2-7. ISSN 0006-3525. PMID 12209474. https://pubmed.ncbi.nlm.nih.gov/12209474.
- ↑ Ohuchi, Masaki; Murakami, Hiroshi; Suga, Hiroaki (2007). "The flexizyme system: a highly flexible tRNA aminoacylation tool for the translation apparatus". Current Opinion in Chemical Biology 11 (5): 537–542. doi:10.1016/j.cbpa.2007.08.011. PMID 17884697.
- ↑ Katoh, Takayuki; Iwane, Yoshihiko; Suga, Hiroaki (2017-12-15). "Logical engineering of D-arm and T-stem of tRNA that enhances d-amino acid incorporation" (in en). Nucleic Acids Research 45 (22): 12601–12610. doi:10.1093/nar/gkx1129. ISSN 0305-1048. PMID 29155943.
- ↑ Iqbal, Emil S.; Dods, Kara K.; Hartman, Matthew C. T. (2018). "Ribosomal incorporation of backbone modified amino acids via an editing-deficient aminoacyl-tRNA synthetase" (in en). Organic & Biomolecular Chemistry 16 (7): 1073–1078. doi:10.1039/c7ob02931d. ISSN 1477-0539. PMID 29367962.
 |
