Chemistry:2-Imidazolidinethione
From HandWiki
| |

| |
| Names | |
|---|---|
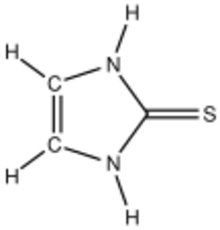
| Preferred IUPAC name
1,5-Dihydro-2H-imidazole-2-thione | |
| Other names
2-Mercaptoimidazole
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H4N2S | |
| Molar mass | 100.14 g·mol−1 |
| Appearance | white solid |
| Melting point | 225–227 °C (437–441 °F; 498–500 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Ethylene thiourea, Aminothiazole, 1,3-Dihydroimidazol-2-one (2-hydroxyimidazole) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Imidazolidinethione is the organosulfur compound with the formula C2H2(NH)2C=S. It is a cyclic unsaturated thiourea with a short C=S bond length of 169 pm.[1] The compound is often referred to as 2-mercaptoimidazole, which is a tautomer that is not observed. The compound forms a variety of metal complexes.[2] In terms of bonding and reactivity, 2-imidazolidinethione is similar to mercaptobenzimidazole.
References
- ↑ Owczarzak, Agata; Dutkiewicz, Zbigniew; Kurczab, Rafał; Pietruś, Wojciech; Kubicki, Maciej; Grześkiewicz, Anita M. (2019). "Role of Staple Molecules in the Formation of S···S Contact in Thioamides: Experimental Charge Density and Theoretical Studies". Crystal Growth & Design 19 (12): 7324–7335. doi:10.1021/acs.cgd.9b01204.
- ↑ Lobana, Tarlok S.; Sharma, Renu; Sharma, Rekha; Butcher, Ray J. (2008). "Metal Derivatives of Heterocyclic Thioamides: Synthesis and Crystal Structures of Copper Complexes with 1-Methyl-1,3-imidazoline-2-thione and 1,3-Imidazoline-2-thione". Zeitschrift für Anorganische und Allgemeine Chemie 634 (10): 1785–1790. doi:10.1002/zaac.200800161.
 |

