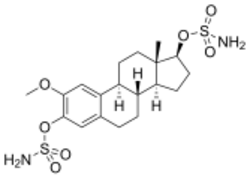
Chemistry:2-Methoxyestradiol disulfamate
 | |
| Clinical data | |
|---|---|
| Other names | 2-Methoxyestradiol disulfamate; 2-Methoxyestradiol 3,17β-disulfamate; 2-Methoxyestradiol-3,17β-O,O-bis(sulfamate); 3,17β-bis-Sulfamoyloxy-2-methoxyestra-1,3,5(10)-triene |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C19H28N2O7S2 |
| Molar mass | 460.56 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
2-Methoxyestradiol disulfamate (developmental code STX-140; also known as 2-methoxyestradiol 3,17β-O,O-bis(sulfamate)) is a synthetic, oral active anti-cancer medication which was previously under development for potential clinical use.[1][2] It has improved potency, low metabolism, and good pharmacokinetic properties relative to 2-methoxyestradiol (2-MeO-E2).[3] It is also a potent inhibitor of steroid sulfatase, the enzyme that catalyzes the desulfation of steroids such as estrone sulfate and dehydroepiandrosterone sulfate (DHEA-S).
2-Methoxyestradiol disulfamate exhibits anti-angiogenic activity and induction of cell cycle arrest and apoptosis in human tumor xenografts, with clinical potential for hormone–independent tumors. Some of this activity stems from tubulin binding at the colchicine site and disruption of interphase microtubules. 2-Methoxyestradiol disulfamate is highly active in tumors that are resistant to chemotherapy.[4]
In xenograft models of breast and prostate cancer complete cures were achieved after oral treatment with 2-methoxyestradiol disulfamate and drug-resistant tumors also shrank in size after oral treatment.[2] Conventional treatments for hormone-independent cancers targeting tubulin are associated with side effects, such as neurotoxicity, and can only be given infrequently and intravenously. 2-Methoxyestradiol disulfamate is more effective on the same tumors, blocks metastatic spread without the peripheral neuropathy associated with current clinical anticancer drugs.[5]
See also
References
- ↑ "2-substituted estradiol bis-sulfamates, multitargeted antitumor agents: synthesis, in vitro SAR, protein crystallography, and in vivo activity". Journal of Medicinal Chemistry 49 (26): 7683–7696. December 2006. doi:10.1021/jm060705x. PMID 17181151.
- ↑ 2.0 2.1 "Estrogen O-sulfamates and their analogues: Clinical steroid sulfatase inhibitors with broad potential". The Journal of Steroid Biochemistry and Molecular Biology 153 (19): 160–169. September 2015. doi:10.1016/j.jsbmb.2015.03.012. PMID 25843211.
- ↑ "Discovery and Development of the Aryl O-Sulfamate Pharmacophore for Oncology and Women's Health". Journal of Medicinal Chemistry 58 (19): 7634–7658. October 2015. doi:10.1021/acs.jmedchem.5b00386. PMID 25992880.
- ↑ "STX140 is efficacious in vitro and in vivo in taxane-resistant breast carcinoma cells". Clinical Cancer Research 14 (2): 597–606. January 2008. doi:10.1158/1078-0432.CCR-07-1717. PMID 18223236.
- ↑ "STX140, but not paclitaxel, inhibits mammary tumour initiation and progression in C3(1)/SV40 T/t-antigen transgenic mice". PLOS ONE 8 (12): e80305. 2013-12-06. doi:10.1371/journal.pone.0080305. PMID 24324595. Bibcode: 2013PLoSO...880305M.
 |

